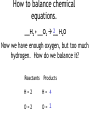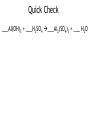* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download How to balance chemical equations.
Chemical warfare wikipedia , lookup
Destruction of Syria's chemical weapons wikipedia , lookup
Rate equation wikipedia , lookup
Fine chemical wikipedia , lookup
History of molecular theory wikipedia , lookup
Atomic theory wikipedia , lookup
Drug discovery wikipedia , lookup
California Green Chemistry Initiative wikipedia , lookup
Chemical equilibrium wikipedia , lookup
History of chemistry wikipedia , lookup
Transition state theory wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical Corps wikipedia , lookup
Chemical plant wikipedia , lookup
Determination of equilibrium constants wikipedia , lookup
Chemical industry wikipedia , lookup
Stoichiometry wikipedia , lookup
Detailed balance wikipedia , lookup
How to balance chemical equations. •When pieces of matter come together or break apart, it is called a reaction. •The law of conservation of matter states that matter can not be created or destroyed. •In any reaction, you must have the same amount of each part before and after the reaction happens. •We show how reactions become balanced by using chemical equations. How to balance chemical equations. 2Na + Cl2 2NaCl This is a balanced chemical equation. How to balance chemical equations. 2Na + Cl2 2NaCl The arrow is called the yields sign. It separates what you start with on the left from what you end up with on the right. How to balance chemical equations. 2Na + Cl2 2NaCl To the left of the yields sign are the parts that react. They are called the reactants. How to balance chemical equations. 2Na + Cl2 2NaCl To the right of the yields sign is what you end up with after the reaction. These are called the products. How to balance chemical equations. 2Na + Cl2 2NaCl The little number next to and below an element is called a subscript. We have been using them all along. We never change subscripts in a chemical equation. How to balance chemical equations. 2Na + Cl2 2NaCl The big numbers next to the elements or compounds are called coefficients. These are the only things we can change in a chemical equation. How to balance chemical equations. 2Na + Cl2 2NaCl Coefficients tell us the amount of each compound or element we have. You multiply the subscript for each element to the right of a coefficient until you are stopped by a + or How to balance chemical equations. 2Na + Cl2 2NaCl So in this equation you have 2 x 1 sodium atoms being added to 2 chlorine atoms yielding 2 x 1 sodium chloride molecules. How to balance chemical equations. ___H2 + ___O2 ___H2O When we balance chemical equations, we need to figure out what coefficients to use. How to balance chemical equations. ___H2 + ___O2 ___H2O To do that, we list how many atoms of each element we start with, then use math to figure out the coefficients. How to balance chemical equations. ___H2 + ___O2 ___H2O In this equation, we start with: Reactants Products H=2 H=2 O=2 O=1 How to balance chemical equations. ___H2 + ___O2 ___H2O Our hydrogen is balanced, but we don’t have enough oxygen in the product. Reactants Products H=2 H=2 O=2 O=1 How to balance chemical equations. ___H2 + ___O2 ___H2O We need to make our best mathematical guess as to which coefficient will bring balance to the equation. Reactants Products H=2 H=2 O=2 O=1 How to balance chemical equations. 2 H2O ___H2 + ___O2 ___ We need two oxygen atoms, so lets try a coefficient of 2 in front of the product. Reactants Products H=2 H=2 O=2 O=1 How to balance chemical equations. 2 H2O ___H2 + ___O2 ___ Multiply that 2 by the subscripts for each atom in the product. Reactants Products H=2 H= 4 O=2 O= 2 How to balance chemical equations. 2 H2O ___H2 + ___O2 ___ Now we have enough oxygen, but too much hydrogen. How do we balance it? Reactants Products H=2 H= 4 O=2 O= 2 How to balance chemical equations. 2___H2 + ___O2 ___ 2 H2O If we put a coefficient of 2 in front of the H2 on the reactants side, we can balance the equation. Reactants Products H= 4 H= 4 O-2 O= 2 How to balance chemical equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coefficients to your equation. •If there are poly-atomic ions on both sides of your equation, don’t separate them! Quick Check ___Al(OH)3 + ___H2SO4 ___Al2(SO4)3 + ___ H2O