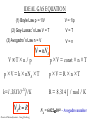* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemical Thermodynamics : Georg Duesberg
Chemical warfare wikipedia , lookup
Fluorochemical industry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Computational chemistry wikipedia , lookup
Chemical industry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Atomic theory wikipedia , lookup
Stoichiometry wikipedia , lookup
Chemical weapon wikipedia , lookup
Gas chromatography wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical potential wikipedia , lookup
Chemical Corps wikipedia , lookup
Industrial gas wikipedia , lookup
History of chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical Thermodynamics : Georg Duesberg The Properties of Gases Kinetic gas theory – Maxwell Boltzman distribution, Collisions Real (non-ideal) gases – fugacity, Joule Thomson effect Mixtures of gases – Entropy, Chemical Potential Liquid Solutions - Electrolyte activity, Henry’s and Raoult’s Law Thermodynamics of Mixtures - Colligative Properties Chemical Thermodynamics : Georg Duesberg Readings • • • • Atkins - PC Mortimer – PC Pitzer and Brewer – Thermodynamics Kittel Kroemer - Thermal Physics http://www.tcd.ie/Chemistry/staff/people/duesberg/ASIN/ 20web/2027-10-09/teaching.html Or also via my chemistry staff page - link to ASIN page Chemical Thermodynamics : Georg Duesberg Chapter 1 The Properties of Gases Chemical Thermodynamics : Georg Duesberg Pressure pressure = force area p=F/A Ar molecules/atoms of gas are constantly in motion Chemical Thermodynamics : Georg Duesberg Kinetic theory and Gas Laws the pressure of a gas increases when it is compressed at constant temperature? – Boyles Law • When a gas is compressed at constant temperature, • the molecules have less volume to move and hit the wall of the container more frequently. • As a result, pressure will increases. Chemical Thermodynamics : Georg Duesberg Kinetic theory and Gas Laws Boyle’s Law pressure – volume relationship (temperature is constant) Boyle (1627-1691) Chemical Thermodynamics : Georg Duesberg p ∞ 1/V Kinetic theory and Gas Laws The volume of a gas increases when heated at constant pressure - Charles’ Law • When a gas is heated, the gas molecules move faster and • hit the wall of the container violently. • The volume of gas must increase to keep the pressure constant. • So that the gas molecules hit the wall less frequently. Chemical Thermodynamics : Georg Duesberg Gay-Lussac’s Law (also Charles law) temperature – volume relationship (pressure is constant) Gay-Lussac (1778-1850) V∞T Chemical Thermodynamics : Georg Duesberg Kinetic theory and Gas Laws ISOTHERMS p ∞ 1/V p = const/V => p × V = const p1 × V1 = const p2 × V2 = const The pressure-volume dependence of a fixed amount of perfect gas at different temperatures. Each curve is a hyperbola (pV = constant) and is called an isotherm. Chemical Thermodynamics : Georg Duesberg p 1 × V1 = p 2 × V 2 Kinetic theory and Gas Laws The pressure of a fixed volume of gas increases with temperature. • As temperature rises, the molecules move faster • The molecules will hit the walls of the container frequently and violently • Hence, the pressure increases Chemical Thermodynamics : Georg Duesberg Isobare V∞T V = const’ ×T V/T = const’ V1 / T1 = const’ V2 / T2 = const The variation of the volume of a fixed amount of gas with the temperature constant. Note that in each case they extrapolate to zero volume at -273.15° C. Chemical Thermodynamics : Georg Duesberg V1 / T1 = V2 / T2 Surface of states Isobare and Isotherm Chapter 1 : Slide 12 Chemical Thermodynamics : Georg Duesberg Avogadro’s Law Avogadro (1776-1856) 2 H2(g) + O2(g) → 2 H2O(l) R = 8.314 J / mol / K k=1.38X10-23J/K NA = 6.022×1023 – Avogadro number Chemical Thermodynamics : Georg Duesberg n∞V n 1 / V1 = n 2 / V 2 N Ak = R Avogadro principle: Volume of real gases At a given T and p, equal volumes of gases contain the same number of molecules, Vm = V/n. Table below presents the molar volumes of selected gases at standard conditions (SATP 25°C and 100kPa) 3 Vm/(dm Perfect gas 24.7896* Ammonia 24.8 Argon 24.4 Carbon dioxide 24.6 Nitrogen 24.8 Oxygen 24.8 Hydrogen 24.8 Helium 24.8 At STP Vm = 22.414 m3/kmol at 0 °C and 101.325 kPa dm3 mol-1. At IUPAC = 22.711 m3/kmol at 0 °C and 100 kPa m3/kmol Chemical Thermodynamics : Georg Duesberg 1 mol− ) Gas useful : p1V1 p 2 V2 = n1T1 n 2 T2 IDEAL GAS EQUATION (1) Boyle Law p ∞ 1/V V ∞ 1/p (2) Gay-Lussac’s Law V ∞ T V∞T (3) Avogadro’s Law n ∞ V V ∞T × n / p V∞n N = nNA p × V = const × n × T p × V = k × nNA × T p ×V = R × n ×T k=1.38X10-23J/K R = 8.314 J / mol / K N Ak = R Chemical Thermodynamics : Georg Duesberg NA = 6.022×1023 – Avogadro number Application: Barometric formula: p as a function of height Consider a column of gas with unit cross sectional area. Chemical Thermodynamics : Georg Duesberg Variation of pressure with altitude Barometric formula: p as a function of height Boundary condition: ground level pressure is p0 so that p = p0 exp(-Mgh/RT) An exponential decrease of p with height. Equal Δh's always give the same proportional change in p. Note the assumptions: 1) Ideal gas behavior 2) Constant g 3) Isothermal atmosphere Mgh is the gravitational potential energy. We will often see properties varying in proportion to exp(-E/RT) = exp(-ε/kBT) where E is a form of molar energy (ε is a molecular energy) because these are examples of "Boltzmann distributions". Chapter 1 : Slide 17 Chemical Thermodynamics : Georg Duesberg Barometric Formula As elevation increases, the height of the atmosphere decreases and its pressure decreases. F = mg = ρVg = ρhSg F ρghS P= = = ρgh S S m N = 2 2 s m mass moles (M W ) = volume V Rewrite PV = nRT as Therefore, Chemical Thermodynamics : Georg Duesberg kg m kg x x m = m3 s 2 m2 density = ρ = Write in differential form. dP = − ρ gdh Check units. PM W ρ= RT n P = V RT Continue Derivation of Barometric Formula Substitute the expression for density into the differential eqn. PM W g dP = − dh RT Divide both sides of the above equation by P and integrate. ⌠ dP = −⌠ M W g dh ⎮ ⎮ ⌡ RT ⌡ P Integration of the left side and moving the constants outside the integral on the right side of the differential equation gives, MW g MW g ln P = − dh = − h + ln C ∫ RT RT Chemical Thermodynamics : Georg Duesberg Continue Derivation of Barometric Formula Evaluating the integral between the limits of P0 at zero height and Ph at height h, gives The constant of integration C can be determined from the initial condition P(h = 0) = P0, where P0 is the average sea level atmospheric pressure. − M W gh ln Ph = + ln P0 RT Ph = P0 e Chemical Thermodynamics : Georg Duesberg M W gh − RT Sample calculation • Calculate the pressure on Mount Carrauntoohil (1,038 m) under normal conditions? h = 1082 m Temperature as 25°C T = 298 K P0 = 101.3 kPa = 1 bar (760 Torr) m(air) = 29 g/mol (N2 = 28 amu, O2 =32 amu) g = 9.81 ms-2 Standard gravity R = 8.314 J / mol / K p = p0 exp(-mgh/RT) = 89.5 kPa = 671 Torr 21 Chemical Thermodynamics : Georg Duesberg IG-09 Height distribution in a gas • Energy (E = Mgy) being considered is significantly higher than a quanta of energy. E is nearly continuous. • Easier to think of probability density functions: P ( x, x + dx; x, y + dy; x, z + dz ) ∝ e − mgy kT dxdydz P is the probability of finding a molecule between x & x + dx, y & y + dy and z & z + dz NB: dx, dy and dz are large compared to a molecule but small compared to the size of the system Chemical Thermodynamics : Georg Duesberg 22 Probability density function e The directions parallel to the ground (x & z) do not contribute to the probability density function; only the height (y) above ground has an influence dy P ( y) ∝ mgy − kT dxdydz y dx x Chemical Thermodynamics : Georg Duesberg Height distribution in a gas P ( y) ∝ e mgy − kT dxdydz For an ideal gas at constant temperature T, the probability density P(y) is related to the number density (# of molecules N per unit volume V ) n(y) : n( y) = n ( y = 0) Chemical Thermodynamics : Georg Duesberg e mgy − kT DALTON’S LAW pure gases gas mixtures (atmospheres) the total pressure of a gas mixture, p, is the sum of the pressures of the individual gases (partial pressures) at a Dalton (1801) Chemical Thermodynamics : Georg Duesberg constant temperature and volume p = pA + pB + pC + …. p×V=n×R×T pA = n A × R × T / V pB = n B × R × T / V p = (nA + nB) × R × T / V mole fraction x < 1 pA / p = nA /(nA + nB) = xA p A = xA × p n p = Σ pi i=1 Chemical Thermodynamics : Georg Duesberg p = pA + p B Dalton’s Law Suppose we have two gases in a container: nA moles of gas A and nB moles of gas B. We can define individual partial pressures pA = nART/V and pB = nBRT/V . Dalton’s Law is that the measured total pressure p is the sum of the partial pressures of all the components: p = pA+pB+… = (nA+nB+…)RT/V. Mole fractions: define xJ for species J as nJ/n where n = (nA +nB+…). Then, xA + xB + … = 1 and pJ = p xJ Chemical Thermodynamics : Georg Duesberg Chapter 2 Kinetic gas theory Chemical Thermodynamics : Georg Duesberg Kinetic Molecular Theory of Gases macroscopic (gas cylinder) microscopic Maxwell (1831-1879) Chemical Thermodynamics : Georg Duesberg (atoms/molecules) Boltzmann (1844-1906) Kinetic Molecular Theory of Gases Physical properties of gases can be described by motion of individual gas atoms/molecules Assumptions: 1) each macroscopic and microscopic particle in motion holds an kinetic energy according to Newton’s law 2) They undergo elastic collisions 3) They are large in number and are randomly distributed 4) They can be treated as points of mass (diameter<< mean free path) Chemical Thermodynamics : Georg Duesberg Kinetic Molecular Theory of Gases: Assumptions 1) According to Newton's law of action–reaction, the force on the wall is equal in magnitude to this value, but oppositely directed. 2.) Elastic collision with wall: -‐v vafter = -vbefore v Δvelocity 2v Force = mass × =m Δtime Δt Kinetic Molecular Theory of Gases: Assumptions 3. Avogardo Number – Brownian motion 4. Gases are composed of atoms/molecules which are separated from each other by a distance l much more than their own diameter d d = 10-10 m L = 10-3 m….. few m molecules are mass points with negligible volume Collisions of the gas molecules with a wall L Freaction Chemical Thermodynamics : Georg Duesberg Small volume, v=LA, adjacent to wall where L is less than the mean free path As a result of a collision with the wall the momentum of a molecule changes by Kinetic Molecular Theory of Gases Pressure = Forcetotal/Area P=F/A • Ftotal = F1 collision x number of collisions in a particular time interval Only molecules within a distance νxΔt with νx > 0 can reach the wall on the right in an interval Δt. L = vx Δt Assume that in a time Δt every molecule (atom) in the original volume, v=LA, within the range of velocities will collide with the wall. Chemical Thermodynamics : Georg Duesberg Collisions of the gas molecules with a wall This means that Δt is given by: The “reaction force” of a molecule on the wall is the negative of the average rate of change in the momentum of gas molecules in the volume v that collide with the wall in the time Δt. The total force on the wall is the sum of the average rate of momentum change for all molecules in the volume v=LA that collide with the wall Here we have divided by 2 since only ½ of the molecules in our volume have a positive velocity toward the wall Chemical Thermodynamics : Georg Duesberg Collisions of the gas molecules with a wall (cont.) We do the sum by noting that the total number of molecules in the volume v is (N/ V) v=LA N/ V = density Remembering Pascal’s law dividing by A yields the pressure everywhere. P=F L Chemical Thermodynamics : Georg Duesberg A Kinetic theory: go from 1 to 3 dimensions L Velocity squared of a molecule: 2 2 v = vx + v y + vz The average of a sum is equal to the sum of averages… All the directions of motion (x, y, z) are equally probable. Remember homogeneous Equipartition principle and isotropic! Chemical Thermodynamics : Georg Duesberg 2 2 Kinetic theory Combing these results yields From the ideal gas law And with c = <v> v 2 3kT 2 3kT =c= = 2m m Relation between the absolute temperature and average kinetic energy of a molecule. Chemical Thermodynamics : Georg Duesberg Kinetic theory vrms of a molecule is “thermal speed”: The absolute temperature is a measure of the average kinetic energy of a molecule. Example: What is the thermal speed of hydrogen molecules at 800K? Chemical Thermodynamics : Georg Duesberg