Stoichiometric Calculations
... Limiting reagent problems are worded differently because the quantities of both reactants are given. 10 moles of H2 and 20 moles of Cl2 react to produce HCl. Which quantity is the limiting reagent? It is your job to figure out which reactant is limiting because that will determine the maximum amount ...
... Limiting reagent problems are worded differently because the quantities of both reactants are given. 10 moles of H2 and 20 moles of Cl2 react to produce HCl. Which quantity is the limiting reagent? It is your job to figure out which reactant is limiting because that will determine the maximum amount ...
Stoichiometric Calculations
... Stoichiometry Calculations with Volumes At a given temperature and pressure, the space a sample of a gas takes up (it's volume) is proportional to the number of moles of gas molecules present. Therefore... 2 H2 (g) ...
... Stoichiometry Calculations with Volumes At a given temperature and pressure, the space a sample of a gas takes up (it's volume) is proportional to the number of moles of gas molecules present. Therefore... 2 H2 (g) ...
enjoy chemistry
... The elements present in Group 18 have their valence shell orbitals completely filled and, therefore, react with a few elements only under certain conditions. Therefore, they are now known as noble gases. (ii)Noble gases are mostly chemically inert. Their inertness to chemical reactivity is attribute ...
... The elements present in Group 18 have their valence shell orbitals completely filled and, therefore, react with a few elements only under certain conditions. Therefore, they are now known as noble gases. (ii)Noble gases are mostly chemically inert. Their inertness to chemical reactivity is attribute ...
Natural gas sweetening using ionic liquids
... natural gas liquefying, and also as emissions. It was observed that the solubility of aliphatic hydrocarbons in [emim][FAP] is relatively low, thus they will remain in the natural gas stream. This is an advantage because these compounds have a high heating value and they can be liquefied for various ...
... natural gas liquefying, and also as emissions. It was observed that the solubility of aliphatic hydrocarbons in [emim][FAP] is relatively low, thus they will remain in the natural gas stream. This is an advantage because these compounds have a high heating value and they can be liquefied for various ...
Unit 2: Matter as Solutions and Gases
... 5. Only H+, NH4+, Na+, K+ cations with PO43−, SO32− and CO32− are soluble (exception Li2CO3 is soluble). 6. Only H+, NH4+, Li+, Na+, K+, Ni2+, Zn2+ cations with IO3− and OOCCOO2− are soluble (exceptions: Co(IO3)2 and Fe2(OOCCOO)3 are soluble). 7. Only H+, NH4+, Li+, Na+, K+, Mg2+, Ca2+ cations with ...
... 5. Only H+, NH4+, Na+, K+ cations with PO43−, SO32− and CO32− are soluble (exception Li2CO3 is soluble). 6. Only H+, NH4+, Li+, Na+, K+, Ni2+, Zn2+ cations with IO3− and OOCCOO2− are soluble (exceptions: Co(IO3)2 and Fe2(OOCCOO)3 are soluble). 7. Only H+, NH4+, Li+, Na+, K+, Mg2+, Ca2+ cations with ...
Electrospun Polyaniline Fibers as Highly Sensitive Room
... promising candidates as chemiresistive sensor materials.[7-9] The unique combination of high specific surface area, mechanical flexibility, room temperature operation, low cost of fabrication, and large range of conductivity change makes these materials particularly attractive as nanoscale resistanc ...
... promising candidates as chemiresistive sensor materials.[7-9] The unique combination of high specific surface area, mechanical flexibility, room temperature operation, low cost of fabrication, and large range of conductivity change makes these materials particularly attractive as nanoscale resistanc ...
EDEXCEL A LeveL - Hodder Education
... Scientists have the concept of an ‘ideal gas’ which obeys the gas laws perfectly. In practice, real gases do not obey the laws under all conditions. Under laboratory conditions, however, there are gases which are close to behaving like an ideal gas. These are the gases which, at room temperature, ar ...
... Scientists have the concept of an ‘ideal gas’ which obeys the gas laws perfectly. In practice, real gases do not obey the laws under all conditions. Under laboratory conditions, however, there are gases which are close to behaving like an ideal gas. These are the gases which, at room temperature, ar ...
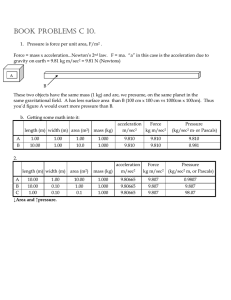
book problems c 10.
... in 1010. The relative atomic mass of silicon is particularly important, since silicon crystals are used in the x-ray methods mentioned above. As a continuation of this approach, one of the 1999 NIST Precision Measurement Grants has been awarded to David Pritchard, physics professor at the Massachuse ...
... in 1010. The relative atomic mass of silicon is particularly important, since silicon crystals are used in the x-ray methods mentioned above. As a continuation of this approach, one of the 1999 NIST Precision Measurement Grants has been awarded to David Pritchard, physics professor at the Massachuse ...
Topic 1 Quantitative Chemistry File
... ratio of atoms of each element in a particle of a substance. Formula, molecular: The formula showing the actual number of atoms of each element in a particle of a substance. Formula, structural: Shows the arrangement of atoms and bonds within a molecule. Ideal gas: A gas for which the relationship p ...
... ratio of atoms of each element in a particle of a substance. Formula, molecular: The formula showing the actual number of atoms of each element in a particle of a substance. Formula, structural: Shows the arrangement of atoms and bonds within a molecule. Ideal gas: A gas for which the relationship p ...
CHAPTER 11
... bloodstream. The body does not metabolize nitrogen, however, so it can accumulate in a diver’s body during a dive. The extra nitrogen can affect the nerve cells of the diver, causing nitrogen narcosis. Divers suffering from nitrogen narcosis become disoriented and experience symptoms similar to into ...
... bloodstream. The body does not metabolize nitrogen, however, so it can accumulate in a diver’s body during a dive. The extra nitrogen can affect the nerve cells of the diver, causing nitrogen narcosis. Divers suffering from nitrogen narcosis become disoriented and experience symptoms similar to into ...
Chemistry Worksheets
... Classify the following as either elements, compounds, homogeneous mixtures (solutions) or heterogeneous mixtures: ...
... Classify the following as either elements, compounds, homogeneous mixtures (solutions) or heterogeneous mixtures: ...
1 Quantitative chemistry - Pearson Schools and FE Colleges
... Chemistry was a late developer as a physical science. Newton was working on the laws of physics more than a century before the work of the French chemist Antoine Lavoisier (1743–1794) brought chemistry into the modern age. Chemical reactions involve changes in smell, colour and texture and these are ...
... Chemistry was a late developer as a physical science. Newton was working on the laws of physics more than a century before the work of the French chemist Antoine Lavoisier (1743–1794) brought chemistry into the modern age. Chemical reactions involve changes in smell, colour and texture and these are ...
Chemistry Standard Level Chapter 1
... Chemistry was a late developer as a physical science. Newton was working on the laws of physics more than a century before the work of the French chemist Antoine Lavoisier (1743–1794) brought chemistry into the modern age. Chemical reactions involve changes in smell, colour and texture and these are ...
... Chemistry was a late developer as a physical science. Newton was working on the laws of physics more than a century before the work of the French chemist Antoine Lavoisier (1743–1794) brought chemistry into the modern age. Chemical reactions involve changes in smell, colour and texture and these are ...
Absorption of Flue-Gas Components by Ionic Liquids
... Enthalpy of fusion and heat capacity: Enthalpy of fusion (Hfus) and heat capacity (Cp) were measured for SO2 absorption with a DSC 111 SETARAM calorimeter. The temperature of the thermostat was measured by the resistance of a suitable Pt resistor, which was calibrated by the melting points of stand ...
... Enthalpy of fusion and heat capacity: Enthalpy of fusion (Hfus) and heat capacity (Cp) were measured for SO2 absorption with a DSC 111 SETARAM calorimeter. The temperature of the thermostat was measured by the resistance of a suitable Pt resistor, which was calibrated by the melting points of stand ...
Chapter 9 slides
... Gases consist of particles (atoms or molecules) that are relatively far apart. Gas particles move about rapidly. An average O2 molecule moves at a velocity of 980 mi/hr at room temperature. Gas particles have little effect on one another unless they collide. When they collide, they do not stick to ...
... Gases consist of particles (atoms or molecules) that are relatively far apart. Gas particles move about rapidly. An average O2 molecule moves at a velocity of 980 mi/hr at room temperature. Gas particles have little effect on one another unless they collide. When they collide, they do not stick to ...
ض ( ا ء ا ط ك ا رر 108 1) -
... Rank the atoms Br, Cl, and K in order of increasing electronegativity? C Br < Cl < K K < Br < Cl B Cl < Br < K D K < Cl < Br Anions are formed when a neutral atom gains one or more electrons ...
... Rank the atoms Br, Cl, and K in order of increasing electronegativity? C Br < Cl < K K < Br < Cl B Cl < Br < K D K < Cl < Br Anions are formed when a neutral atom gains one or more electrons ...
Industrial gas

Industrial gases are a group of gases that are specifically manufactured for use in a wide range of industries, which include oil and gas, petrochemicals, chemicals, power, mining, steelmaking, metals, environmental protection, medicine, pharmaceuticals, biotechnology, food, water, fertilizers, nuclear power, electronics and aerospace. Their production is a part of the wider chemical Industry (where industrial gases are often seen as ""speciality chemicals"").The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene; although a huge variety of gases and mixtures are available in gas cylinders.The industry producing these gases is known as the industrial gases industry, which is seen as also encompassing the supply of equipment and technology to produce and use the gases.Whilst most industrial gas is usually only sold to other industrial enterprises; retail sales of gas cylinders and associated equipment to tradesmen and the general public are available through gas local agents and typically includes products such as balloon helium , dispensing gases for beer kegs, welding gases and welding equipment, LPG and medical oxygen. Very small scale gas supply is not confined to just the industrial gas companies. A wide variety of hand-carried small gas containers, which may be called cylinders, bottles, cartridges, capsules or canisters are available to supply LPG, butane, propane, carbon dioxide or nitrous oxide. Examples are whippets, powerlets, campingaz and sodastream.