Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
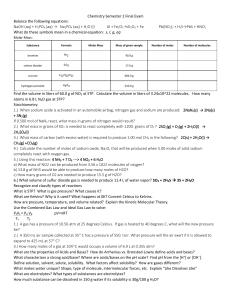
... 3.) What mass of carbon (with excess water) is required to produce 1.00 mol CH4 in the following? 2C(s) + 2H2O(l) → CH4(g) +CO2(g) 24.02 g 4.) Calculate the number of moles of sodium oxide, Na2O, that will be produced when 5.00 moles of solid sodium completely react with oxygen gas. 2.5 mol 5.) Usin ...
... 3.) What mass of carbon (with excess water) is required to produce 1.00 mol CH4 in the following? 2C(s) + 2H2O(l) → CH4(g) +CO2(g) 24.02 g 4.) Calculate the number of moles of sodium oxide, Na2O, that will be produced when 5.00 moles of solid sodium completely react with oxygen gas. 2.5 mol 5.) Usin ...
ap unit 5 worksheet answers
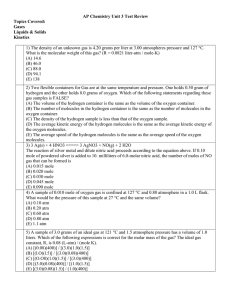
... 3. A fixed quantity of gas at 23⁰C exhibits a pressure of 748 torr and occupies a volume of 10.3 L. Calculate the volume the gas will occupy if the temperature is increased to 145 ⁰C 15 L 4. A fixed quantity of gas at 21⁰C exhibits a pressure of 748 torr and occupies a volume of 10.3 L. Calculate th ...
... 3. A fixed quantity of gas at 23⁰C exhibits a pressure of 748 torr and occupies a volume of 10.3 L. Calculate the volume the gas will occupy if the temperature is increased to 145 ⁰C 15 L 4. A fixed quantity of gas at 21⁰C exhibits a pressure of 748 torr and occupies a volume of 10.3 L. Calculate th ...
Unit 2
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
Unit 2
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
... Welcome to Advanced Placement Chemistry. AP Chem is a fast paced course, with higher orders of thinking. You will be expected to pull on previous knowledge constantly to solve problems. Along the way, we will see some fun demonstrations and perform some intense chemical experiments. The attached sum ...
1 - gcisd
... 30. Water has several unique properties such as high boiling point, high surface tension and low vapor pressure. What are the main causes for these properties? 31. What happens at the triple point on a phase diagram? 32. Like dissolves like refers to the molecule’s _________________________. 33. Wha ...
... 30. Water has several unique properties such as high boiling point, high surface tension and low vapor pressure. What are the main causes for these properties? 31. What happens at the triple point on a phase diagram? 32. Like dissolves like refers to the molecule’s _________________________. 33. Wha ...
CHEM 2: Exam 3
... 15. Identify the strongest intermolecular force present in the covalent substance hydrazine with the formula N 2H4. A. London Dispersion Forces B. Dipole-Dipole Forces C. Hydrogen Bonding D. Inter-ionic Forces E. Not enough information is provided: the Lewis dot structure and connections between the ...
... 15. Identify the strongest intermolecular force present in the covalent substance hydrazine with the formula N 2H4. A. London Dispersion Forces B. Dipole-Dipole Forces C. Hydrogen Bonding D. Inter-ionic Forces E. Not enough information is provided: the Lewis dot structure and connections between the ...
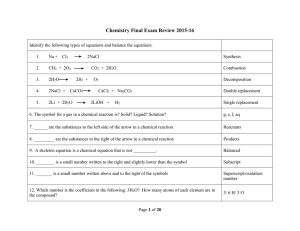
Type Of Chemical Reaction
... g. You start with a saturated solution of NH3 in 100 grams of water at 10 °C. How many grams of NH3 gas would bubble out of the solution if you raise the temperature to 90 °C? approx 60 grams 70g-10g=60g h. What is the independent variable on this graph? Temperature i. What is the dependent variable ...
... g. You start with a saturated solution of NH3 in 100 grams of water at 10 °C. How many grams of NH3 gas would bubble out of the solution if you raise the temperature to 90 °C? approx 60 grams 70g-10g=60g h. What is the independent variable on this graph? Temperature i. What is the dependent variable ...
worksheer format 11-12
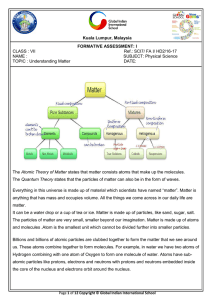
... For example, an electric current can be passed through water to form the elements hydrogen and oxygen. Water can be decomposed into hydrogen and oxygen. Water is a compound made up of hydrogen and oxygen. Types of mixtures ...
... For example, an electric current can be passed through water to form the elements hydrogen and oxygen. Water can be decomposed into hydrogen and oxygen. Water is a compound made up of hydrogen and oxygen. Types of mixtures ...
AP Chemistry Unit 3 Test Review Topics Covered: Gases Liquids
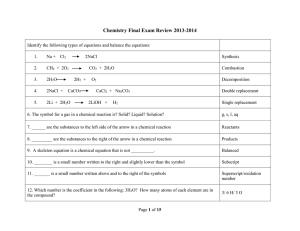
... Short Response: No calculator maybe used. For each of the following three reactions, in part (i) write a balanced equation for the reaction and in part (ii) answer the question about the reaction. In part (i), coefficients should be in terms of lowest whole numbers. Assume that solutions are aqueous ...
... Short Response: No calculator maybe used. For each of the following three reactions, in part (i) write a balanced equation for the reaction and in part (ii) answer the question about the reaction. In part (i), coefficients should be in terms of lowest whole numbers. Assume that solutions are aqueous ...
Type Of Chemical Reaction
... 7. Solubility Curve: Use the following curve to answer questions a. Which of the curves in this figure represents a gas? NH3 b. Which substance is the most soluble at 50 °C? KI c. How many grams of KNO3 are needed in order to make a saturated solution of KNO3 in 100 g of H2O at 50 °C? 80 g ...
... 7. Solubility Curve: Use the following curve to answer questions a. Which of the curves in this figure represents a gas? NH3 b. Which substance is the most soluble at 50 °C? KI c. How many grams of KNO3 are needed in order to make a saturated solution of KNO3 in 100 g of H2O at 50 °C? 80 g ...
Introductory Chemistry: A Foundation FOURTH EDITION by Steven
... 10. Repeat – A trick of the trade, when you are forced to attack an element that is in 3 or more compounds – find where it is uncombined. You can find a factor to make it any amount you want, even if that factor is a fraction! – We want to make the O on the left equal 5, therefore we will multiply i ...
... 10. Repeat – A trick of the trade, when you are forced to attack an element that is in 3 or more compounds – find where it is uncombined. You can find a factor to make it any amount you want, even if that factor is a fraction! – We want to make the O on the left equal 5, therefore we will multiply i ...
Science 9
... Phase describes a PHYSICAL state of matter. Matter “moves” from one phase to another by physical forces such as temperature and pressure. If energy is added (e.g., increased temperature) or taken away (e.g., freezing), a physical change is created. SOLID + e = LIQUID + e = GAS + e = PLASMA Any kind ...
... Phase describes a PHYSICAL state of matter. Matter “moves” from one phase to another by physical forces such as temperature and pressure. If energy is added (e.g., increased temperature) or taken away (e.g., freezing), a physical change is created. SOLID + e = LIQUID + e = GAS + e = PLASMA Any kind ...
quant6stoichiom
... ex. How many moles of ammonia are produces by 2.8 mol of hydrogen gas? set up as ratio 2 mol NH3: 3 mol H2 = n mole NH3: 2.8 mol H2 ...
... ex. How many moles of ammonia are produces by 2.8 mol of hydrogen gas? set up as ratio 2 mol NH3: 3 mol H2 = n mole NH3: 2.8 mol H2 ...
Industrial gas

Industrial gases are a group of gases that are specifically manufactured for use in a wide range of industries, which include oil and gas, petrochemicals, chemicals, power, mining, steelmaking, metals, environmental protection, medicine, pharmaceuticals, biotechnology, food, water, fertilizers, nuclear power, electronics and aerospace. Their production is a part of the wider chemical Industry (where industrial gases are often seen as ""speciality chemicals"").The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene; although a huge variety of gases and mixtures are available in gas cylinders.The industry producing these gases is known as the industrial gases industry, which is seen as also encompassing the supply of equipment and technology to produce and use the gases.Whilst most industrial gas is usually only sold to other industrial enterprises; retail sales of gas cylinders and associated equipment to tradesmen and the general public are available through gas local agents and typically includes products such as balloon helium , dispensing gases for beer kegs, welding gases and welding equipment, LPG and medical oxygen. Very small scale gas supply is not confined to just the industrial gas companies. A wide variety of hand-carried small gas containers, which may be called cylinders, bottles, cartridges, capsules or canisters are available to supply LPG, butane, propane, carbon dioxide or nitrous oxide. Examples are whippets, powerlets, campingaz and sodastream.