* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Analgetics - TMA Department Sites
Time perception wikipedia , lookup
Signal transduction wikipedia , lookup
Proprioception wikipedia , lookup
Metastability in the brain wikipedia , lookup
Perception of infrasound wikipedia , lookup
Neuroregeneration wikipedia , lookup
Neuroanatomy wikipedia , lookup
Molecular neuroscience wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Microneurography wikipedia , lookup
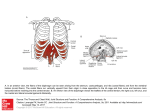
Narcotics and Analgesics Pain Universal, complex, subjective experience Number one reason people take medication Generally is related to some type of tissue damage and serves as a warning signal Scope of the Problem Increases as Baby Boomers age 25 million people suffer acute pain related to surgery or injury Chronic pain affects 250 million Americans Is a multibillion dollar industry Much ignorance exists about this complaint Gate Control Theory of Pain Gate control theory of pain is the idea that physical pain is not a direct result of activation of pain receptor neurons, but rather, its perception is modulated by interaction between different neurons Gate Control Theory of Pain Nerve fibers (A delta (fast channels)) and C fibers (slow channels) transmit pain impulses from the periphery Impulses are intercepted in the dorsal horns of the spinal cord, the substantia gelatinosa In this region, cells can be inhibited or facilitated to the T-cells (trigger cells) Gate Control Theory of Pain cont. When cells in the substantia gelatinosa are inhibited, the ‘gate’ to the brain is closed When facilitated, the ‘gate’ to the brain is open Gate Control Theory of Pain Similar gating mechanisms exist in the nerve fibers descending from the thalamus and the cortex. These areas of the brain regulate thoughts and emotions. Thus, with a pain stimulus, one’s thoughts and emotions can actually modify the pain experience. Pathophysiological Response Tissue damage activates free nerve endings (nociceptors) of peripheral nerves Pain signal is transmitted to the spinal cord, hypothalamus, and cerebral cortex Pain is transmitted to spinal cord by A-delta fibers and C fibers Pathophysiological Response A-delta fibers transmit fast, sharp, well-localized pain signals C fibers conduct the pain signal slowly and produce poorly localized, dull, or burning type of pain Thalamus is the relay station for incoming stimuli, incl. pain Pain Fibers and Pathways A delta fibers found in the skin and muscle, myelinated, respond to mechanical stimuli. Produce intermittent pain. C fibers distributed in the muscle as well as the periosteum and the viscera. These fibers are unmyelinated, conduct thermal, chemical and strong mechanical stimuli. Produce persistent pain. Inhibitory and Facilitatory Mechanisms Neurotransmitters—chemicals that exert inhibitory or excitatory activity at postsynaptic nerve cell membranes. Examples include: acetylcholine, norepinehprine, epinephrine, dopamin, and serotonin. Neuromodulators—endogenous opiates. Hormones in brain. Alpha endorphins, beta endorphins and enkephalins. Help to relieve pain. Opioid Receptors Opioid receptors—binding sites not only for endogenous opiates but also for opioid analgesics to relieve pain. Several types of receptors: Mu, Kappa, Delta, Epsilon and Sigma. Mu Receptors Location: CNS incl. brainstem, limbic system, dorsal horn of spinal cord Morphine sulfate and morphine sulfate agonists bind to Mu receptors Sources of Pain Nociceptive—free nerve endings that receive painful stimuli Neuropathic –damaged nerves Narcotic Analgesics Relieve moderate to severe pain by inhibiting release of Substance P in central and peripheral nerves; reducing the perception of pain sensation in brain, producting sedation and decreasing emotional upsets associated with pain Narcotic Analgesics Can be given orally, IM, sub q, IV or even transdermally Orally are metabolized by liver, excreted by kidney—caution if compromised Morphine and meperidine produce metabolites Widespread effects: CNS, Resp., GI Narcotics—Mechanisms of Action Bind to opioid receptors in brain and SC and even in periphery Indications for Use Before and during surgery Before and during invasive diagnostic procedures During labor and delivery Tx acute pulmonary edema Treating severe, nonproductive cough Contraindications to Use Respiratory depression Chronic lung disease Chronic liver or kidney disease BPH Increased intracranial pressure Hypersensitivity reactions Changing Philosophy on Pain Undermedicated Titrate to comfort Management Considerations age-specific considerations Morphine often drug of choice—nonceiling. Other nonceiling drugs include: hydromorphone, levorphanol and methadone Use non-narcotic when able Combinations may work by different mechanisms thus greater efficacy (e.g. Tylenol w/codeine) Route selections Oral preferred IV most rapid—PCA allows self administration. Basal dosage. More effective, requires less dosing. Epidural, intrathecal or local injection Can use rectal suppositories or transdermal routes Dosage Dosages of narcotic analgesics should be reduced for clients receiving other CNS depressants such as other sedative-type drugs, antihistamines or sedating antianxiety medications Scheduling Give narcotics before encouraging turning, coughing and deep breathing in post-surgical patients Automatic stop orders after 72h In acute pain, narcotic analgesics are most effective when given parenterally and at start of pain Individual Drugs Agonists have activity on mu and kappa opioid receptors Agonist/antagonists have agonist activity in some receptors; antagonists in others. Have lower abuse potential than pure agonists; because of antagonism—can produce withdrawal symptoms Antagonists are antidote drugs Agonists Alfenta (alfentanil)—short duration Codeine Sublimaze or Duragesic (Fentanyl)—short duration Dilaudid (hydromorphone) Demerol (meperidine)—preferred in urinary and biliary colic, less resp. depression newborns Morphine OxyContin Agonists cont. Darvon (propoxyphene) Ultram (tramadol) Methadone Agonists/Antagonists Have lower abuse potential than pure agonists Buprenex (buprenorphine) Nubain (nalbuphine) Talwin (pentazocine) Stadol (butohanol)—also in nasal spray Antagonists Revex (nalmefene)—longer duration of action than Narcan Narcan (naloxone) ReVia (naltrexone)-used in maintenance of opiate-free states in opiate addicts