* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2.1 The Nature of Matter - Sonoma Valley High School
Metastable inner-shell molecular state wikipedia , lookup
Computational chemistry wikipedia , lookup
Coordination complex wikipedia , lookup
Organic chemistry wikipedia , lookup
Atomic orbital wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Periodic table wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Electronegativity wikipedia , lookup
Biochemistry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Livermorium wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
History of chemistry wikipedia , lookup
Aromaticity wikipedia , lookup
Chemical element wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Isotope analysis wikipedia , lookup
Molecular dynamics wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Homoaromaticity wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Metallic bonding wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Electron configuration wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Isotopic labeling wikipedia , lookup
Extended periodic table wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Atomic nucleus wikipedia , lookup
Chemical bond wikipedia , lookup
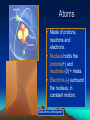
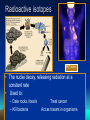
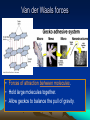
2.1 The Nature of Matter Atoms • Made of protons, neutrons and electrons. • Nucleus holds the protons(+) and neutrons (0) + mass. • Electrons (-) surround the nucleus, in constant motion. Gold atoms: photograph Elements and Isotopes 24+ elements are found in living things (of 100+ elements) Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. Radioactive isotopes PET scan • The nuclei decay, releasing radiation at a constant rate • Used to: – Date rocks, fossils – Kill bacteria Treat cancer Act as tracers in organisms Compounds NaCl H2O • A substance formed by joining atoms together. • The smallest unit of a compound is a molecule. • Formulas: tell types of elements in the compound and their ratios. Chemical bonds • Hold atoms together, forming molecules • Ionic bonds: electrons transfer from one atom to another, creating a charged particle, or ion • Covalent bonds: atoms share electrons • Water is a covalently bonded molecule found in all living things Water molecules are Attracted to one another Van der Waals forces • Forces of attraction between molecules. • Hold large molecules together. • Allow geckos to balance the pull of gravity. These synthetic Carbon nanotubes make a ‘glue’ 10X stickier than a gecko’s foot