History
... new element. In 1895 Ramsay discovered helium in the uranium mineral clevite while it was independently discovered in cleveite by the Swedish chemists Cleve and Langlet at about the same time. Rutherford and Royds in 1907 demonstrated that alpha particles are helium nuclei. ...
... new element. In 1895 Ramsay discovered helium in the uranium mineral clevite while it was independently discovered in cleveite by the Swedish chemists Cleve and Langlet at about the same time. Rutherford and Royds in 1907 demonstrated that alpha particles are helium nuclei. ...
4.1 Defining the Atom
... You can think through this problem using four simple ideas about matter and electric charges. 1. Atoms have no net electric charge; they are electrically neutral. 2. Electric charges are carried by particles of matter. 3. Electric charges always exist in whole-number multiples of a single basic unit ...
... You can think through this problem using four simple ideas about matter and electric charges. 1. Atoms have no net electric charge; they are electrically neutral. 2. Electric charges are carried by particles of matter. 3. Electric charges always exist in whole-number multiples of a single basic unit ...
A. Alpha particle. - MDC Faculty Web Pages
... • are emitted when a nucleus in an excited state moves to a lower energy state • are more harmful than alpha or beta particles • are most penetrating because they have no mass or charge • are pure energy, greater per photon than in visible or ultraviolet light and X-rays • are unaffected by magnetic ...
... • are emitted when a nucleus in an excited state moves to a lower energy state • are more harmful than alpha or beta particles • are most penetrating because they have no mass or charge • are pure energy, greater per photon than in visible or ultraviolet light and X-rays • are unaffected by magnetic ...
Chemistry Atoms, Molecules, and Ions 2.1 Multiple
... B) Rutherford's gold foil experiment. C) Thomson's cathode ray tube experiment. D) None of these Answer: B Topic: Section 2.4 Atomic Structure: Protons and Neutrons 18) The existence of neutrons in the nucleus of an atom was demonstrated by A) Millikan's oil drop experiment. B) Rutherford's gold foi ...
... B) Rutherford's gold foil experiment. C) Thomson's cathode ray tube experiment. D) None of these Answer: B Topic: Section 2.4 Atomic Structure: Protons and Neutrons 18) The existence of neutrons in the nucleus of an atom was demonstrated by A) Millikan's oil drop experiment. B) Rutherford's gold foi ...
Preview Sample 2
... 21. Which of the following is the same for isotopes of an element? A. mass number B. mass of an atom C. neutron number D. atomic number E. both atomic number and neutron number 22. Which of the following statements about isotopes is incorrect? A. The isotopes of an element have the same number of pr ...
... 21. Which of the following is the same for isotopes of an element? A. mass number B. mass of an atom C. neutron number D. atomic number E. both atomic number and neutron number 22. Which of the following statements about isotopes is incorrect? A. The isotopes of an element have the same number of pr ...
DOE FUNDAMENTALS HANDBOOK NUCLEAR PHYSICS AND REACTOR THEORY Volume 1 of 2
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
Into the Atom - Structure of the Nucleus, Heavy Nuclei... Original Script and research: Dr. Arvind Dubey
... to become attached to nuclei. After this few of our ancestral nuclei were combined in stars through the process of nuclear fusion to produce more of the element helium, and the sequence of elements from carbon up to iron. Almost all the atoms present on Earth today were present in the nebula from wh ...
... to become attached to nuclei. After this few of our ancestral nuclei were combined in stars through the process of nuclear fusion to produce more of the element helium, and the sequence of elements from carbon up to iron. Almost all the atoms present on Earth today were present in the nebula from wh ...
chemistry - My Study materials – Kumar
... KVS RO Bangalore and respected sir Shri M.K. Kulshreshtha, Retd-AC, KVS RO Chandigarh for their blessings, motivation and encouragement in bringing out this project in such an excellent form. I also extend my special thanks to respected sir Shri. P. S. Raju, Principal, KV Gachibowli, respected madam ...
... KVS RO Bangalore and respected sir Shri M.K. Kulshreshtha, Retd-AC, KVS RO Chandigarh for their blessings, motivation and encouragement in bringing out this project in such an excellent form. I also extend my special thanks to respected sir Shri. P. S. Raju, Principal, KV Gachibowli, respected madam ...
Nuclear Physics 1 NWNC
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
... their reactor-specific content, DOE Category A reactor training managers also reviewed and commented on the content. On the basis of feedback from these sources, information that applied to two or more DOE nuclear facilities was considered generic and was included. The final draft of each of the han ...
OC 583- ISOTOPE BIGEOCHEMISTRY
... equal number of neutrons and protons (e.g. 12C6, 14N7, 16O8, 32S16) (Fig. 6) -but not always: (e.g. 7Li3, 9Be4) 3. Since isotopes have the same number of protons and electrons this means isotopes have same chemical behavior, i.e., all isotopic species enter into same chemical reactions and form the ...
... equal number of neutrons and protons (e.g. 12C6, 14N7, 16O8, 32S16) (Fig. 6) -but not always: (e.g. 7Li3, 9Be4) 3. Since isotopes have the same number of protons and electrons this means isotopes have same chemical behavior, i.e., all isotopic species enter into same chemical reactions and form the ...
Isotopes of Volatile Organic Compounds: An Emerging Approach for
... and the mechanism of the molecule’s production. The element can also be fractionated by physical, chemical, or biological loss processes. Isotope fractionation occurs because the bond energy of each isotope is slightly different, with heavier isotopes having stronger bonds and typically slower react ...
... and the mechanism of the molecule’s production. The element can also be fractionated by physical, chemical, or biological loss processes. Isotope fractionation occurs because the bond energy of each isotope is slightly different, with heavier isotopes having stronger bonds and typically slower react ...
Chm 1
... ____ 14. All isotopes of hydrogen contain a. one neutron. c. one proton. b. two electrons. d. two nuclei. ____ 15. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus of an oxygen atom. b. oxygen nuclides. c. neutrons outside the oxygen atom's nucleus. d. energy ...
... ____ 14. All isotopes of hydrogen contain a. one neutron. c. one proton. b. two electrons. d. two nuclei. ____ 15. The atomic number of oxygen, 8, indicates that there are eight a. protons in the nucleus of an oxygen atom. b. oxygen nuclides. c. neutrons outside the oxygen atom's nucleus. d. energy ...
FREE Sample Here
... A. Odd number of neutrons and odd number of protons B. Even number of neutrons and odd number of protons C. Odd number of neutrons and even number of protons D. Even number of neutrons and even number of protons E. None of the options above results in significantly more stable nuclei. ...
... A. Odd number of neutrons and odd number of protons B. Even number of neutrons and odd number of protons C. Odd number of neutrons and even number of protons D. Even number of neutrons and even number of protons E. None of the options above results in significantly more stable nuclei. ...
Chapter 2 - Atoms and the Periodic Table (test bank)
... probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have equal numbers of protons and electrons) and that the mass of a proton is significantly greater than the mass ...
... probe the structure of the atoms. He observed that most of these alpha particles penetrated the foil and were not deflected. Realizing that atoms are electrically neutral (that is, they have equal numbers of protons and electrons) and that the mass of a proton is significantly greater than the mass ...
Isotope

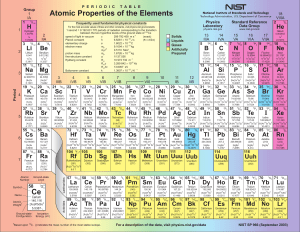
Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.