FREE Sample Here
... B. The atom also contains 12 electrons. C. The atom also contains 23 electrons. D. There are no other factors related to the 11 protons and 12 neutrons. Section 2.4 Difficulty Level: Easy 17. Atoms of elements belonging to the same group have an identical number of A. total electrons. B. energy leve ...
... B. The atom also contains 12 electrons. C. The atom also contains 23 electrons. D. There are no other factors related to the 11 protons and 12 neutrons. Section 2.4 Difficulty Level: Easy 17. Atoms of elements belonging to the same group have an identical number of A. total electrons. B. energy leve ...
FREE Sample Here
... B. The atomic weights include protons and neutrons at 1 amu each, but they also include electrons, which weigh a lot less than one. C. The atomic weight is the weighted average of the masses of the known isotopes of an element. D. The atomic weights do not include isotopes. Section 2.4 Difficulty Le ...
... B. The atomic weights include protons and neutrons at 1 amu each, but they also include electrons, which weigh a lot less than one. C. The atomic weight is the weighted average of the masses of the known isotopes of an element. D. The atomic weights do not include isotopes. Section 2.4 Difficulty Le ...
nuclear physics - Thierry Karsenti
... to abide by the recommendations made on the basis of the mark obtained by the learner. As their instructor you should encourage learners to evaluate themselves by answering all the questions provided below. Education research shows that this will help learners be more prepared and help them articula ...
... to abide by the recommendations made on the basis of the mark obtained by the learner. As their instructor you should encourage learners to evaluate themselves by answering all the questions provided below. Education research shows that this will help learners be more prepared and help them articula ...
FREE Sample Here
... A. The proton has the atomic mass of 1 amu. B. The proton has the same charge as the neutron. C. The proton has greater mass than an electron. D. The proton and the neutron have approximately the same atomic mass. Section 2.1 Difficulty Level: Easy 3. The atom’s structure characteristically has A. t ...
... A. The proton has the atomic mass of 1 amu. B. The proton has the same charge as the neutron. C. The proton has greater mass than an electron. D. The proton and the neutron have approximately the same atomic mass. Section 2.1 Difficulty Level: Easy 3. The atom’s structure characteristically has A. t ...
FREE Sample Here
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
general-organic-and-biological-chemistry-3rd-edition
... C. Electrons have greater mass than protons. D. Neutrons are found in the nucleus of the atom. Section 2.1 Difficulty Level: Easy 2. Choose the incorrect statement about the proton: A. The proton has the atomic mass of 1 amu. B. The proton has the same charge as the neutron. C. The proton has greate ...
... C. Electrons have greater mass than protons. D. Neutrons are found in the nucleus of the atom. Section 2.1 Difficulty Level: Easy 2. Choose the incorrect statement about the proton: A. The proton has the atomic mass of 1 amu. B. The proton has the same charge as the neutron. C. The proton has greate ...
Preview Sample 1
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
... 14) Krypton-81m is used for lung ventilation studies. Its half-life is 13 seconds. How long does it take the activity of this isotope to reach one-quarter of its original value? Answer: 1 → 1/2 → 1/4, so that is two half lives; therefore, 26 secs Diff: 2 Section: 2-6 15) In order for a radionuclide ...
Textbook sample chapter
... named the tiny particles atoms. His scientific idea was that atoms are indivisible and indestructible. All atoms of an element are identical and have the same mass and chemical properties. Atoms of different elements have different masses (he called them atomic weights) and different chemical proper ...
... named the tiny particles atoms. His scientific idea was that atoms are indivisible and indestructible. All atoms of an element are identical and have the same mass and chemical properties. Atoms of different elements have different masses (he called them atomic weights) and different chemical proper ...
High School Knowledge Exam – Study Guide
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
Atoms: The Building Blocks of Matter
... negative electric charge. And because cathode rays have identical properties regardless of the element used to produce them, it was concluded that electrons are present in atoms of all elements. Thus, cathode-ray experiments provided evidence that atoms are divisible and that one of the atom’s basic ...
... negative electric charge. And because cathode rays have identical properties regardless of the element used to produce them, it was concluded that electrons are present in atoms of all elements. Thus, cathode-ray experiments provided evidence that atoms are divisible and that one of the atom’s basic ...
Chapter 2 – Atoms, Ions, and the Periodic Table
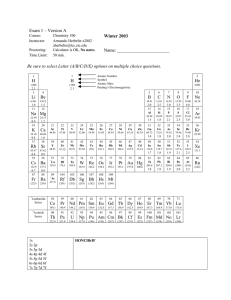
... (a) From the periodic table you find that the element with an atomic number of 1 is hydrogen, H. Since the isotope has 2 neutrons, the mass number is 3. The isotope symbol for hydrogen-3 is 13 H . (b) The element with an atomic number of 4 is beryllium, Be. Since the isotope has 5 neutrons, the mass ...
... (a) From the periodic table you find that the element with an atomic number of 1 is hydrogen, H. Since the isotope has 2 neutrons, the mass number is 3. The isotope symbol for hydrogen-3 is 13 H . (b) The element with an atomic number of 4 is beryllium, Be. Since the isotope has 5 neutrons, the mass ...
Ch 2 Atoms and Elements Student
... Dalton’s Atomic Theory 1. Each element is made up of tiny indestructible particles called atoms. 2. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements 3. Atoms combine in simple whole number ratios to form compounds. 4. Atoms o ...
... Dalton’s Atomic Theory 1. Each element is made up of tiny indestructible particles called atoms. 2. All atoms of a given element have the same mass and other properties that distinguish them from atoms of other elements 3. Atoms combine in simple whole number ratios to form compounds. 4. Atoms o ...
Atoms, Elements, and
... English scientist. He conducted experiments using a cathode ray tube, which is a glass tube sealed at both ends out of which most of the air has been pumped. Thomson’s tube had a metal plate at each end. The plates were connected to a high-voltage electrical source that gave one of the plates—the an ...
... English scientist. He conducted experiments using a cathode ray tube, which is a glass tube sealed at both ends out of which most of the air has been pumped. Thomson’s tube had a metal plate at each end. The plates were connected to a high-voltage electrical source that gave one of the plates—the an ...
Chapter 4 and 5
... Demo mole amounts of various elements? Note how they are NOT the same amount!! ...
... Demo mole amounts of various elements? Note how they are NOT the same amount!! ...
The Atomic Theory
... these substances A and B. It turned out that, given the same amount of carbon, forming B always required exactly twice as much oxygen as forming A. In other words, if you could make A with 3 grams of carbon and 4 grams of oxygen, B could be made with the same 3 grams of carbon but with 8 grams of ox ...
... these substances A and B. It turned out that, given the same amount of carbon, forming B always required exactly twice as much oxygen as forming A. In other words, if you could make A with 3 grams of carbon and 4 grams of oxygen, B could be made with the same 3 grams of carbon but with 8 grams of ox ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.