39 The Atomic Nucleus and Radioactivity
... • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occu ...
... • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occu ...
39 The Atomic Nucleus and Radioactivity
... • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occu ...
... • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occu ...
39 The Atomic Nucleus and Radioactivity
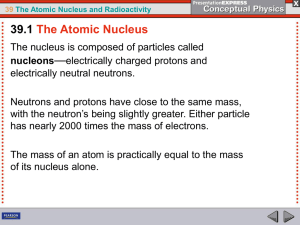
... As we know, within the nucleus, there is a repulsive force between protons that are in the nucleus. (Like charges repel.) Stability of an atom, therefore, is due to a “balance” between the strong force’s tendency to hold the nucleus together and the electrical force’s tendency to blow it apart. A nu ...
... As we know, within the nucleus, there is a repulsive force between protons that are in the nucleus. (Like charges repel.) Stability of an atom, therefore, is due to a “balance” between the strong force’s tendency to hold the nucleus together and the electrical force’s tendency to blow it apart. A nu ...
F:\Users\Steven\Documents\Chemistry\CHEM120\Problem Set
... When I went to write this problem I looked at the periodic table and saw that Rubidium had a mass of 85.467. Since the mass of all isotopes are even (or nearly so) and this average was uneven I knew immediately that rubidium had to have two major isotopes. When I looked up the isotopes sure enough t ...
... When I went to write this problem I looked at the periodic table and saw that Rubidium had a mass of 85.467. Since the mass of all isotopes are even (or nearly so) and this average was uneven I knew immediately that rubidium had to have two major isotopes. When I looked up the isotopes sure enough t ...
Depleted and enriched mantle sources for Paleo- and
... (far exceeding the crustal levels) further buffer them against any possible effects of crustal contamination (Bell et al., 1982; Bell and Blenkinsop, 1987a; Tilton and Bell, 1994). Although carbonatites are typically associated with alkaline silicate rocks and preferentially emplaced through contine ...
... (far exceeding the crustal levels) further buffer them against any possible effects of crustal contamination (Bell et al., 1982; Bell and Blenkinsop, 1987a; Tilton and Bell, 1994). Although carbonatites are typically associated with alkaline silicate rocks and preferentially emplaced through contine ...
Stoichiometry
... 6. Compound X2Y is 60% X by mass. Calculate the percent Y by mass of the compound XY3? If you have 100 g of X2Y there would be 60g of X and 40g of Y For XY3 since it has only one X atom you can think of that as ½(60g) or 30 g of X and since there’s three Y atoms you can think of that as 3(40g) or 12 ...
... 6. Compound X2Y is 60% X by mass. Calculate the percent Y by mass of the compound XY3? If you have 100 g of X2Y there would be 60g of X and 40g of Y For XY3 since it has only one X atom you can think of that as ½(60g) or 30 g of X and since there’s three Y atoms you can think of that as 3(40g) or 12 ...
Nitrogen and Oxygen Family
... be stable. Trihalides except BiF3 are predominantly covalent in nature. Halides are hydrolysed in water forming oxyacids or oxychlorides. PCl3 + H2O H3PO3 + HCl SbCl3 + H2O SbOCl (orange) + 2HCl BiCl3 + H2O BiOCl (white) + 2HCl NITROGEN (N) : Dinitrogen comprises 78% of the earth atm ...
... be stable. Trihalides except BiF3 are predominantly covalent in nature. Halides are hydrolysed in water forming oxyacids or oxychlorides. PCl3 + H2O H3PO3 + HCl SbCl3 + H2O SbOCl (orange) + 2HCl BiCl3 + H2O BiOCl (white) + 2HCl NITROGEN (N) : Dinitrogen comprises 78% of the earth atm ...
chemical and isotopic evidence for the in situ origin of marine humic
... and from soils, These acids are shown to be major components of the organic matter from marine and nonmarine sediments-some marine sediments may contain 70% of their organic carbon in the hmnic and fulvic acid fraction. Marine and terrestrial humic acids have similar carbon and hydrogen content, but ...
... and from soils, These acids are shown to be major components of the organic matter from marine and nonmarine sediments-some marine sediments may contain 70% of their organic carbon in the hmnic and fulvic acid fraction. Marine and terrestrial humic acids have similar carbon and hydrogen content, but ...
CHEM 121 Chp 5 Spaulding
... A chemical change (a chemical reaction) converts one substance into another ◦ Breaking bonds in the reactants (starting materials) ◦ Forming new bonds in the products ...
... A chemical change (a chemical reaction) converts one substance into another ◦ Breaking bonds in the reactants (starting materials) ◦ Forming new bonds in the products ...
File - Mrs. Roy`s Science Class
... 3. If you are asked to find that amount in grams/molecules, convert moles to the unit you are asked to find. ...
... 3. If you are asked to find that amount in grams/molecules, convert moles to the unit you are asked to find. ...
Pathways for Nitrogen Cycling in Earth`s Crust and Upper Mantle: A
... The percentage of N in the mantle is more difficult to estimate but it is thought to be near 60%, ...
... The percentage of N in the mantle is more difficult to estimate but it is thought to be near 60%, ...
chapter - Max-Planck-Institut für Astronomie
... Our story reads that once upon a time, it existed an interstellar cloud of gas and dust. Then, about 4.6 billions years ago, one cloud fragment became the Solar System. What happened to that primordial condensation? When, why and how did it happen? Answering these questions involves putting together ...
... Our story reads that once upon a time, it existed an interstellar cloud of gas and dust. Then, about 4.6 billions years ago, one cloud fragment became the Solar System. What happened to that primordial condensation? When, why and how did it happen? Answering these questions involves putting together ...
FREE Sample Here
... 38. The atomic mass of naturally occurring silver, which is a mixture of two isotopes, is listed as 107.868 u. This means that a. all silver atoms found in nature have a mass which is 107.868/12.000 times as great as that of a 12C atom. b. all silver atoms found in nature have a mass which is 107.86 ...
... 38. The atomic mass of naturally occurring silver, which is a mixture of two isotopes, is listed as 107.868 u. This means that a. all silver atoms found in nature have a mass which is 107.868/12.000 times as great as that of a 12C atom. b. all silver atoms found in nature have a mass which is 107.86 ...
FREE Sample Here
... 38. The atomic mass of naturally occurring silver, which is a mixture of two isotopes, is listed as 107.868 u. This means that a. all silver atoms found in nature have a mass which is 107.868/12.000 times as great as that of a 12C atom. b. all silver atoms found in nature have a mass which is 107.86 ...
... 38. The atomic mass of naturally occurring silver, which is a mixture of two isotopes, is listed as 107.868 u. This means that a. all silver atoms found in nature have a mass which is 107.868/12.000 times as great as that of a 12C atom. b. all silver atoms found in nature have a mass which is 107.86 ...
Chapter 4 and 5
... - truncate if preceding digit is even Why? Long strings of numbers would become very wrong. Go through handout together. HW finish handout ...
... - truncate if preceding digit is even Why? Long strings of numbers would become very wrong. Go through handout together. HW finish handout ...
Chapter 0 A Very Brief History of Chemistry Multiple Choice Questions
... 38. The atomic mass of naturally occurring silver, which is a mixture of two isotopes, is listed as 107.868 u. This means that a. all silver atoms found in nature have a mass which is 107.868/12.000 times as great as that of a 12C atom. b. all silver atoms found in nature have a mass which is 107.86 ...
... 38. The atomic mass of naturally occurring silver, which is a mixture of two isotopes, is listed as 107.868 u. This means that a. all silver atoms found in nature have a mass which is 107.868/12.000 times as great as that of a 12C atom. b. all silver atoms found in nature have a mass which is 107.86 ...
Oxygen diffusion through perovskite membranes
... essentially controlled by two factors, by (i) the rate of the oxygen vacancy diffusion within the membrane and by (ii) the interfacial oxygen exchange on either side of the membrane. The oxygen permeation flux can be increased by reducing the thickness of the membrane, until its thickness becomes le ...
... essentially controlled by two factors, by (i) the rate of the oxygen vacancy diffusion within the membrane and by (ii) the interfacial oxygen exchange on either side of the membrane. The oxygen permeation flux can be increased by reducing the thickness of the membrane, until its thickness becomes le ...
Isotope shift in the electron affinity of chlorine
... lected in a sector magnet and focused by means of several Einzel lenses. In an analyzing chamber, the ions were merged with a laser beam between two electrostatic quadrupole deflectors placed 50 cm apart. An ion current of typically 6 nA for 35 Cl and 2 nA for 37 Cl was measured with a Faraday cup ...
... lected in a sector magnet and focused by means of several Einzel lenses. In an analyzing chamber, the ions were merged with a laser beam between two electrostatic quadrupole deflectors placed 50 cm apart. An ion current of typically 6 nA for 35 Cl and 2 nA for 37 Cl was measured with a Faraday cup ...
Chapter 2 Geochemical Reactions
... controls the chemistry of surface waters and groundwaters. Biological processes play a key role too, and so the bio prefix is often added. In this text, all processes related to the Earth, organic and inorganic are implied in the term geochemistry. These processes include dissolution of air-borne ma ...
... controls the chemistry of surface waters and groundwaters. Biological processes play a key role too, and so the bio prefix is often added. In this text, all processes related to the Earth, organic and inorganic are implied in the term geochemistry. These processes include dissolution of air-borne ma ...
Nucleon number
... 2. All the following statements are true EXCEPT A Nucleus is the positively charged centre of an atom B Protons number indicates the number of protons in an atom C Isotopes are atoms of the same element but with different nucleon number D Nucleon number is the total number of electrons and protons i ...
... 2. All the following statements are true EXCEPT A Nucleus is the positively charged centre of an atom B Protons number indicates the number of protons in an atom C Isotopes are atoms of the same element but with different nucleon number D Nucleon number is the total number of electrons and protons i ...
Chemical Monitoring and Management by Ahmad Shah Idil
... so that it is maintained at a constant 1:3 (N2 to H2). This is because a build-up of any gas must be avoided, as this may dangerously increase the pressure. ...
... so that it is maintained at a constant 1:3 (N2 to H2). This is because a build-up of any gas must be avoided, as this may dangerously increase the pressure. ...
The Effect of Water-Rock Interaction on
... of chemical constituents can be dissolved in groundwater as a result of interaction with the soil and bedrock and from human activities. The variations in groundwater chemistry/quality are discerned from hydrochemical and isotope data. The result reveals that the groundwater chemistry is generally c ...
... of chemical constituents can be dissolved in groundwater as a result of interaction with the soil and bedrock and from human activities. The variations in groundwater chemistry/quality are discerned from hydrochemical and isotope data. The result reveals that the groundwater chemistry is generally c ...
664
... hydrolyzes rapidly in water forming nitric acid and hydrofluoric acid: NO2F + H2O → HNO3 + HF Reaction with ethanol produces ethyl nitrate: NO2F + C2H5OH → C2H5NO3 + HF Reactions with reducing agents can be explosive. The compound attacks most metals almost as vigorously as fluorine. It spontaneousl ...
... hydrolyzes rapidly in water forming nitric acid and hydrofluoric acid: NO2F + H2O → HNO3 + HF Reaction with ethanol produces ethyl nitrate: NO2F + C2H5OH → C2H5NO3 + HF Reactions with reducing agents can be explosive. The compound attacks most metals almost as vigorously as fluorine. It spontaneousl ...
Kinetic isotope effects of 12CH3D+OH and 13CH3D+OH from 278 to
... Abstract. Methane is the second most important long-lived greenhouse gas and plays a central role in the chemistry of the Earth’s atmosphere. Nonetheless there are significant uncertainties in its source budget. Analysis of the isotopic composition of atmospheric methane, including the doubly substi ...
... Abstract. Methane is the second most important long-lived greenhouse gas and plays a central role in the chemistry of the Earth’s atmosphere. Nonetheless there are significant uncertainties in its source budget. Analysis of the isotopic composition of atmospheric methane, including the doubly substi ...
Analytical Techniques for Elemental Analysis of Minerals
... on a micrometer scale. Furthermore, quantitative analytical techniques that can be performed at the micrometer scale are preferred. Even when minerals have larger sizes so that sample amounts of 50 mg or even grams are available, there is another problem to consider: such “bulk samples” may contain ...
... on a micrometer scale. Furthermore, quantitative analytical techniques that can be performed at the micrometer scale are preferred. Even when minerals have larger sizes so that sample amounts of 50 mg or even grams are available, there is another problem to consider: such “bulk samples” may contain ...
Isotope analysis

Isotope analysis is the identification of isotopic signature, the distribution of certain stable isotopes and chemical elements within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level, and subsistence. Variations in isotope ratios from isotopic fractionation are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.The ratios of isotopic oxygen are also differentially affected by global weather patterns and regional topography as moisture is transported. Areas of lower humidity cause the preferential loss of 18O water in the form of vapor and precipitation. Furthermore, evaporated 16O water returns preferentially to the atmospheric system as it evaporates and 18O remains in liquid form or is incorporated into the body water of plants and animals.