* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nuclear Chemistry
Radioactive waste wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear fusion wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Ionizing radiation wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Background radiation wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear fission product wikipedia , lookup
Technetium-99m wikipedia , lookup
Radioactive decay wikipedia , lookup
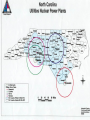
Radioactivity Ch 10 Radioactivity • is the process in which an unstable atomic nucleus emits charged particles & energy • Any atom containing an unstable nucleus is called a radioactive isotope or radioisotope How is the Atom Unstable? • The nuclear “glue” that holds the nucleus together sometimes isn’t strong enough. • Over time the atom “drops” some of it’s subatomic particles …called radioactive decay! 39 19 New Way of Writing Nuclides & Isotopes K ; 4019 K • Superscript is the mass number • Subscript is the atomic number 14 12 • 6C ; 6 C Isotopes • have the same number of p+, different number of no • Another way to show an isotope is to have the mass number follow the name of the element (Carbon-14 or C-14) Types of Nuclear Radiation 1. Alpha 2.Beta 3. Gamma Alpha Decay • Alpha particle—a positively charged particle made up of two p+ & two no • the least penetrating • can be stopped by a sheet of paper Alpha Decay • An alpha particle looks like a helium atom (42He) • mass reduces by 4 • atomic # reduces by 2 • Examples: 238 92 U 209 84 Po Beta Decay • A beta particle is an eemitted by an unstable nucleus • can be stopped by a thin sheet of metal such as aluminum Beta Decay • A beta particle is written 0-1 e • mass remains the same & the atomic # increases by one • Examples: 214 82 Pb 218 84 Po Gamma decay • A gamma ray is a penetrating ray of energy emitted by an unstable nucleus • Gamma rays are energy waves that travel through space at the speed of light Gamma decay • atomic # and mass remain the same, but the energy of nucleus decreases • Gamma rays can be stopped by several centimeters of lead or by several meters of concrete Thanks Cambridge Physics Outlet for amazing graphics! STAR Questions • U-238 loses 4 total subatomic particles, 2 being protons. – What elements are formed? • Thorium and Helium – What type of decay has it undergone? • Alpha decay • What is the most penetrating decay? • Gamma rays Background radiation • is nuclear radiation that occurs naturally in the environment (levels are low enough to be safe) – Radioisotopes in the air, water, rocks, plants, & animals all contribute Background Radiation – Cosmic rays (streams of charged particles) from outer space that collide with the Earth’s atmosphere also contribute – When nuclear radiation exceeds background levels, cells in your body can mutate • Devices used to detect radiation include Geiger counters & film badges Detecting Radiation RATES OF NUCLEAR DECAY A half-life is the time required for one half of a sample of radioactive sample of a radioisotope to decay – Unlike chemical reactions, nuclear decay rates are constant regardless of temperature, pressure or surface area • C-14 has a half life of 5730 years. – What fraction of a sample will be remaining after 1 half life? • After 2 half lives? • C-14 has a half life of 5730 years. – If you have a sample of 50 grams. How much of the sample will be remaining after 1 half life? • After 2 half lives? RATES OF NUCLEAR DECAY Transmutation is the conversion of atoms of one element to atoms of another Transuranium elements are elements with atomic numbers higher than 92 (Uranium) A quark is a subatomic particle theorized to be among the basic units of matter FISSION AND FUSION Fission is the splitting of an atomic nucleus into two smaller parts Fusion is a process in which the nuclei of two atoms combine to form a larger nucleus • A chain reaction is a chain of fission reactions triggered by neutrons released during the fission of a nucleus Fission About 20% of the electricity in the US comes from fission reactions Fission • A tremendous amount of energy is produced during a fission reaction Fission • Advantages: is the lack of air pollution. • Disadvantages: include the risk of exposure & radioactive waste Harris plant, near Raleigh Lake Harris Not in my backyard… Then sealed and transported by way of trucks and trains for more permanent storage. Low-level wastes will stay above ground until they become “stable”. If high-level, the wastes are stored deep underground, where they wait for hundreds to thousands of years to become “stable”. Fusion • release huge amounts of energy • occur in the sun and stars (plasma) Fusion • We do not use fusion reactions for energy b/c of the extremely high temperatures needed to start the reaction & because the plasma would need to be contained. THREE MILE ISLAND • Three Mile Island power station is near Harrisburg, Pennsylvania • In 1979 at Three Mile Island nuclear power plant a cooling malfunction caused part of the core to melt in the # 2 reactor. The TMI-2 reactor was destroyed. • Some radioactive gas was released a couple of days after the accident, but not enough to cause any dose above background levels to local residents. • There were no injuries or adverse health effects from the Three Mile Island accident. CHERNOBYL • The Chernobyl accident in 1986 was the result of a flawed reactor design that was operated with inadequately trained personnel & without proper regard for safety. • The resulting steam explosion & fire released at least five percent of the radioactive reactor core into the atmosphere and downwind. • 28 people died within four months from radiation or thermal burns, 19 have subsequently died, & there have been around nine deaths from thyroid cancer apparently due to the accident: total 56 fatalities as of 2004. • An authoritative UN report in 2000 concluded that there is no scientific evidence of any significant radiationrelated health effects to most people exposed. This was confirmed in a very thorough 2005-06 study. Examples • Iodine-131 has a half-life of 8.07 days. What fraction of a sample of iodine-131 is left unchanged after 16.14 days? Examples • The radioactive isotope Ni-63 has a halflife of 100 yrs. How much of a 10g sample remains after 300 yrs? Examples • How long will it take a sample of Po-194 to decay to 1/8 of its original amount, if Po194 has a half life of 0.7 seconds? Examples • A sample of Cl-38 is observed to decay to 25% of its original amount in 74.4 min. What is the half-life of Cl-38? • sorry…no time for Star cards this time :o)