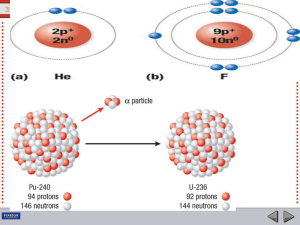
transmutation of nuclides
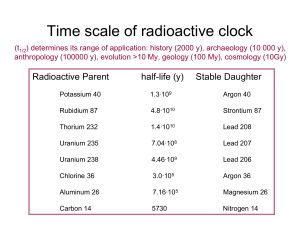
... Actually, the decay of 234 Th to 234Pa is not the end of the decay chain, because 234Pa is a beta emitter with a half life 6.75 hours. In fact, many generations following 234Pa are radioactive and a natural uranium mineral consists of a number of radioactive nuclides. The details of the ...
... Actually, the decay of 234 Th to 234Pa is not the end of the decay chain, because 234Pa is a beta emitter with a half life 6.75 hours. In fact, many generations following 234Pa are radioactive and a natural uranium mineral consists of a number of radioactive nuclides. The details of the ...
Radioactive Decays – transmutations of nuclides
... Actually, the decay of 234 Th to 234Pa is not the end of the decay chain, because 234Pa is a beta emitter with a half life 6.75 hours. In fact, many generations following 234Pa are radioactive and a natural uranium mineral consists of a number of radioactive nuclides. The details of the ...
... Actually, the decay of 234 Th to 234Pa is not the end of the decay chain, because 234Pa is a beta emitter with a half life 6.75 hours. In fact, many generations following 234Pa are radioactive and a natural uranium mineral consists of a number of radioactive nuclides. The details of the ...
This presentation
... ultraviolet and X-rays. They are produced by extreme forces of energy and by atomic decay. The COMPTEL catalog comprises 63 gamma-ray sources. Thirty-two of these are steady sources, such as neutron stars and black hole candidates; the remaining 31 are mysterious gamma-ray bursts, which outshine the ...
... ultraviolet and X-rays. They are produced by extreme forces of energy and by atomic decay. The COMPTEL catalog comprises 63 gamma-ray sources. Thirty-two of these are steady sources, such as neutron stars and black hole candidates; the remaining 31 are mysterious gamma-ray bursts, which outshine the ...
File
... The increasing number of neutrons in the nucleus of the hydrogen atom adds mass to the atom and thus each isotope of a given element has a different mass. Isotopes can be represented as follows: For the isotopes of hydrogen, 1H (or hydrogen-1), 2H (or hydrogen-2) and 3H (or hydrogen-3) represent pro ...
... The increasing number of neutrons in the nucleus of the hydrogen atom adds mass to the atom and thus each isotope of a given element has a different mass. Isotopes can be represented as follows: For the isotopes of hydrogen, 1H (or hydrogen-1), 2H (or hydrogen-2) and 3H (or hydrogen-3) represent pro ...
39 The Atomic Nucleus and Radioactivity
... 39.2 Radioactive Decay Radioactivity is governed by mass-energy equivalence. • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • W ...
... 39.2 Radioactive Decay Radioactivity is governed by mass-energy equivalence. • Particles decay spontaneously only when their combined products have less mass after decay than before. • The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). • W ...
Background radiation

Background radiation is the ubiquitous ionizing radiation that people on the planet Earth are exposed to, including natural and artificial sources.Both natural and artificial background radiation varies depending on location and altitude.