Chm 1
... ____ 54. Which describes particles or electromagnetic radiation emitted from the nucleus during radioactive decay? a. harmless nuclear fallout c. transmutation b. nuclear radiation d. daughter nuclides ____ 55. During radioactive decay, the nucleus disintegrates into a. a lighter and more stable nuc ...
... ____ 54. Which describes particles or electromagnetic radiation emitted from the nucleus during radioactive decay? a. harmless nuclear fallout c. transmutation b. nuclear radiation d. daughter nuclides ____ 55. During radioactive decay, the nucleus disintegrates into a. a lighter and more stable nuc ...
1ST SEM MT CHAP 22 REVIEW
... A controlled-fission chain reaction releases energy in a nuclear reactor. The energy is used to produce steam, which is used to drive the generators that produce the electricity. ...
... A controlled-fission chain reaction releases energy in a nuclear reactor. The energy is used to produce steam, which is used to drive the generators that produce the electricity. ...
39 The Atomic Nucleus and Radioactivity
... mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occur for reactions where more mass results. A proton decaying into a neutron can occur only with external energy input. ...
... mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occur for reactions where more mass results. A proton decaying into a neutron can occur only with external energy input. ...
39 The Atomic Nucleus and Radioactivity
... mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occur for reactions where more mass results. A proton decaying into a neutron can occur only with external energy input. ...
... mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occur for reactions where more mass results. A proton decaying into a neutron can occur only with external energy input. ...
Nuclear Chemistry
... In an α emission the parent element is displaced to a group two places to left and in β emission it will be displaced to a group one place to the right in the periodic table as illustrated in Fig.4.2. This is called Group Displacement Law. It was first stated by Fajans and Soddy (1913) and is often ...
... In an α emission the parent element is displaced to a group two places to left and in β emission it will be displaced to a group one place to the right in the periodic table as illustrated in Fig.4.2. This is called Group Displacement Law. It was first stated by Fajans and Soddy (1913) and is often ...
E = mc 2 - Gordon State College
... Gamma rays: • are high-frequency electromagnetic radiation • are emitted when a nucleus in an excited state moves to a lower energy state • are more harmful than alpha or beta particles • are most penetrating because they have no mass or charge • are pure energy, greater per photon than in visible o ...
... Gamma rays: • are high-frequency electromagnetic radiation • are emitted when a nucleus in an excited state moves to a lower energy state • are more harmful than alpha or beta particles • are most penetrating because they have no mass or charge • are pure energy, greater per photon than in visible o ...
39 The Atomic Nucleus and Radioactivity
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. They will lose their energy after a large number of glancing collisions with atomic electrons. Beta particles slow down until they bec ...
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. They will lose their energy after a large number of glancing collisions with atomic electrons. Beta particles slow down until they bec ...
1002_4th Exam_1010620
... B) The alpha particle is two protons and two neutrons. C) It often leaves the nucleus in an excited state. D) It involves nuclides with atomic number larger than 83 and mass number larger than 200. Answer: A 41) A nuclide has a decay constant of 4.28 × 10-4 /h. If the activity of a sample is 3.14 × ...
... B) The alpha particle is two protons and two neutrons. C) It often leaves the nucleus in an excited state. D) It involves nuclides with atomic number larger than 83 and mass number larger than 200. Answer: A 41) A nuclide has a decay constant of 4.28 × 10-4 /h. If the activity of a sample is 3.14 × ...
Topic 7: Atomic, nuclear and particle physics
... An element’s chemistry is determined by the number of electrons surrounding it. The number electrons an element has is determined by the number of protons in that element’s nucleus. Therefore it follows that isotopes of an element have the same chemical properties. For example there is water, ma ...
... An element’s chemistry is determined by the number of electrons surrounding it. The number electrons an element has is determined by the number of protons in that element’s nucleus. Therefore it follows that isotopes of an element have the same chemical properties. For example there is water, ma ...
Pearson Physics Level 30 Unit VIII Atomic Physics: Chapter 16
... Paraphrase The quantity of mass that is converted to 5.00 GJ of energy is 5.56 108 kg . 5. Isotopes are atoms that have the same atomic number but different neutron numbers. They are chemically very similar but do not have the same atomic mass. 6. A stable nucleus is bound together by the strong ...
... Paraphrase The quantity of mass that is converted to 5.00 GJ of energy is 5.56 108 kg . 5. Isotopes are atoms that have the same atomic number but different neutron numbers. They are chemically very similar but do not have the same atomic mass. 6. A stable nucleus is bound together by the strong ...
p Atomic Structure notes packet 14_15
... 6. Conclusion: Write a rule concerning how to identify an atom from its parts. Review History of the Atom: 1. Thomson’s experiments with cathode ray tube led to the discovery of the _________________. 2. Who was the first person to suggest the idea of atoms in the fourth century B.C.? 3. Who stated ...
... 6. Conclusion: Write a rule concerning how to identify an atom from its parts. Review History of the Atom: 1. Thomson’s experiments with cathode ray tube led to the discovery of the _________________. 2. Who was the first person to suggest the idea of atoms in the fourth century B.C.? 3. Who stated ...
Introduction to Subatomic Physics
... kinetic energy to excitation one. Nuclear reactions (reactions of elementary particles) – nuclear transmutation induced by external action. Change of structure of participated nuclei (particles) and also change of state of motion. Nuclear reactions are also scatterings. Nuclear reactions are possibl ...
... kinetic energy to excitation one. Nuclear reactions (reactions of elementary particles) – nuclear transmutation induced by external action. Change of structure of participated nuclei (particles) and also change of state of motion. Nuclear reactions are also scatterings. Nuclear reactions are possibl ...
MULTIPLE CHOICE. Choose the one alternative that best
... TRUE/FALSE. Write ‘T’ if the statement is true and ‘F’ if the statement is false. 47) Gamma radiation only changes the atomic number but not the mass number of a nucleus. 48) The neutron/proton ratio of stable nuclei increases with increasing atomic number . 49) The energy produced by the sun is the ...
... TRUE/FALSE. Write ‘T’ if the statement is true and ‘F’ if the statement is false. 47) Gamma radiation only changes the atomic number but not the mass number of a nucleus. 48) The neutron/proton ratio of stable nuclei increases with increasing atomic number . 49) The energy produced by the sun is the ...
ch18 - James Goodwin
... The curie is the unit used to express the amount of radioactivity produced by an element. One curie (Ci) = 3.7 x 1010 disintegrations per second. This definition came from the element radium, which has an activity of 1Ci/g Because a curie is so large the millicurie (one thousandth of a curie) and th ...
... The curie is the unit used to express the amount of radioactivity produced by an element. One curie (Ci) = 3.7 x 1010 disintegrations per second. This definition came from the element radium, which has an activity of 1Ci/g Because a curie is so large the millicurie (one thousandth of a curie) and th ...
Absorption and Biological Effects of Ionising Radiation
... Ionising radiation knocks electrons from atoms during its passage, thereby changing their physical state and causing the atoms to become electrically charged, or ionised. The presence of such ions in living tissues has the potential to disrupt normal biological processes. Therefore one objective in ...
... Ionising radiation knocks electrons from atoms during its passage, thereby changing their physical state and causing the atoms to become electrically charged, or ionised. The presence of such ions in living tissues has the potential to disrupt normal biological processes. Therefore one objective in ...
radiometric dating - Tulane University
... Prior to 1905 the best and most accepted age of the Earth was that proposed by Lord Kelvin based on the amount of time necessary for the Earth to cool to its present temperature from a completely liquid state. Although we now recognize lots of problems with that calculation, the age of 25 my was acc ...
... Prior to 1905 the best and most accepted age of the Earth was that proposed by Lord Kelvin based on the amount of time necessary for the Earth to cool to its present temperature from a completely liquid state. Although we now recognize lots of problems with that calculation, the age of 25 my was acc ...
II. Radioactive Decay
... Types of Radiation (cont.) • Gamma rays are high-energy electromagnetic radiation. • Gamma rays have no mass or charge. • Gamma rays almost always accompany alpha and beta radiation. • X rays are a form of high-energy electromagnetic radiation emitted from certain materials in an excited state. (gam ...
... Types of Radiation (cont.) • Gamma rays are high-energy electromagnetic radiation. • Gamma rays have no mass or charge. • Gamma rays almost always accompany alpha and beta radiation. • X rays are a form of high-energy electromagnetic radiation emitted from certain materials in an excited state. (gam ...
radioactive decay - Southwest High School
... Types of Radiation (cont.) • Gamma rays are high-energy electromagnetic radiation. • Gamma rays have no mass or charge. • Gamma rays almost always accompany alpha and beta radiation. • X rays are a form of high-energy electromagnetic radiation emitted from certain materials in an excited state. (gam ...
... Types of Radiation (cont.) • Gamma rays are high-energy electromagnetic radiation. • Gamma rays have no mass or charge. • Gamma rays almost always accompany alpha and beta radiation. • X rays are a form of high-energy electromagnetic radiation emitted from certain materials in an excited state. (gam ...
mass number - Knittig Science
... A nucleon is a general term to denote a nuclear particle - that is, either a proton or a neutron. The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mas ...
... A nucleon is a general term to denote a nuclear particle - that is, either a proton or a neutron. The atomic number Z of an element is equal to the number of protons in the nucleus of that element. The mass number A of an element is equal to the total number of nucleons (protons + neutrons). The mas ...
Chapter 11 Evidence for Strong and Weak Forces in Nuclei
... Nuclear physicists agree that the heaviest nuclei are generally all unstable, because they contain too many neutrons. Such a statement seems to imply that a common method of nuclear decay should be the spontaneous emission of a neutron in order to enable a nucleus to become more stable. However it h ...
... Nuclear physicists agree that the heaviest nuclei are generally all unstable, because they contain too many neutrons. Such a statement seems to imply that a common method of nuclear decay should be the spontaneous emission of a neutron in order to enable a nucleus to become more stable. However it h ...
Is There Any Truth in Modern Physics?
... Nuclear physicists agree that the heaviest nuclei are generally all unstable, because they contain too many neutrons. Such a statement seems to imply that a common method of nuclear decay should be the spontaneous emission of a neutron in order to enable a nucleus to become more stable. However it h ...
... Nuclear physicists agree that the heaviest nuclei are generally all unstable, because they contain too many neutrons. Such a statement seems to imply that a common method of nuclear decay should be the spontaneous emission of a neutron in order to enable a nucleus to become more stable. However it h ...
Slide 1
... mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occur for reactions where more mass results. A proton decaying into a neutron can occur only with external energy input. ...
... mass of a proton plus electron (and the antineutrino). • When a neutron decays, there is less mass. • Decay will not spontaneously occur for reactions where more mass results. A proton decaying into a neutron can occur only with external energy input. ...
Nuclear Chemistry
... • Radioactive decay is the spontaneous disintegration of a nucleus into a slightly lighter nucleus, accompanied by emission of particles, electromagnetic radiation, or both. • Nuclear radiation is particles or electromagnetic radiation emitted from the nucleus during radioactive decay. • An unstable ...
... • Radioactive decay is the spontaneous disintegration of a nucleus into a slightly lighter nucleus, accompanied by emission of particles, electromagnetic radiation, or both. • Nuclear radiation is particles or electromagnetic radiation emitted from the nucleus during radioactive decay. • An unstable ...
Teacher Materials - Scope, Sequence, and Coordination
... distances, are usually stronger than the electric forces that would make it fly apart. Nuclear reactions convert a fraction of the mass of interacting particles into energy, and they can release much greater amounts of energy than atomic interactions. Fission is the splitting of a large nucleus into ...
... distances, are usually stronger than the electric forces that would make it fly apart. Nuclear reactions convert a fraction of the mass of interacting particles into energy, and they can release much greater amounts of energy than atomic interactions. Fission is the splitting of a large nucleus into ...
Radioactive decay
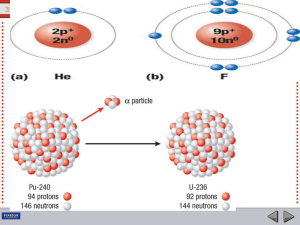
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting radiation. A material that spontaneously emits such radiation — which includes alpha particles, beta particles, gamma rays and conversion electrons — is considered radioactive.Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. The chance that a given atom will decay never changes, that is, it does not matter how long the atom has existed. For a large collection of atoms however, the decay rate for that collection can be calculated from their measured decay constants or half-lives. This is the basis of radiometric dating. The half-lives of radioactive atoms have no known limits for shortness or length of duration, and range over 55 orders of magnitude in time.There are many types of radioactive decay (see table below). A decay, or loss of energy from the nucleus, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element. This is known as a nuclear transmutation.The first decay processes to be discovered were alpha decay, beta decay, and gamma decay. Alpha decay occurs when the nucleus ejects an alpha particle (helium nucleus). This is the most common process of emitting nucleons, but in rarer types of decays, nuclei can eject protons, or in the case of cluster decay specific nuclei of other elements. Beta decay occurs when the nucleus emits an electron or positron and a neutrino, in a process that changes a proton to a neutron or the other way about. The nucleus may capture an orbiting electron, causing a proton to convert into a neutron in a process called electron capture. All of these processes result in a well-defined nuclear transmutation.By contrast, there are radioactive decay processes that do not result in a nuclear transmutation. The energy of an excited nucleus may be emitted as a gamma ray in a process called gamma decay, or be used to eject an orbital electron by its interaction with the excited nucleus, in a process called internal conversion. Highly excited neutron-rich nuclei, formed as the product of other types of decay, occasionally lose energy by way of neutron emission, resulting in a change of an element from one isotope to another. Another type of radioactive decay results in products that are not defined, but appear in a range of ""pieces"" of the original nucleus. This decay, called spontaneous fission, happens when a large unstable nucleus spontaneously splits into two (and occasionally three) smaller daughter nuclei, and generally leads to the emission of gamma rays, neutrons, or other particles from those products.For a summary table showing the number of stable and radioactive nuclides in each category, see radionuclide. There exist twenty-nine chemical elements on Earth that are radioactive. They are those that contain thirty-four radionuclides that date before the time of formation of the solar system, and are known as primordial nuclides. Well-known examples are uranium and thorium, but also included are naturally occurring long-lived radioisotopes such as potassium-40. Another fifty or so shorter-lived radionuclides, such as radium and radon, found on Earth, are the products of decay chains that began with the primordial nuclides, and ongoing cosmogenic processes, such as the production of carbon-14 from nitrogen-14 by cosmic rays. Radionuclides may also be produced artificially in particle accelerators or nuclear reactors, resulting in 650 of these with half-lives of over an hour, and several thousand more with even shorter half-lives. See this list of nuclides for a list of these, sorted by half life.