Bulk Monte Carlo Code Described
... Error! Objects cannot be created from editing field codes. where Vj(r) is the scattering potential of this process, Ek and Ek’ are the initial and final state energies of the particle. The delta function results in conservation of energy for long times after the collision is over, with hω the energy ...
... Error! Objects cannot be created from editing field codes. where Vj(r) is the scattering potential of this process, Ek and Ek’ are the initial and final state energies of the particle. The delta function results in conservation of energy for long times after the collision is over, with hω the energy ...
39 The Atomic Nucleus and Radioactivity
... • When two nucleons are just a few nucleon diameters apart, the nuclear force they exert on each other is nearly zero (small force vectors). • This means that if nucleons are to be held together by the strong force, they must be held in a very small volume. • Nuclei are tiny because the nuclear forc ...
... • When two nucleons are just a few nucleon diameters apart, the nuclear force they exert on each other is nearly zero (small force vectors). • This means that if nucleons are to be held together by the strong force, they must be held in a very small volume. • Nuclei are tiny because the nuclear forc ...
39 The Atomic Nucleus and Radioactivity
... • When two nucleons are just a few nucleon diameters apart, the nuclear force they exert on each other is nearly zero (small force vectors). • This means that if nucleons are to be held together by the strong force, they must be held in a very small volume. • Nuclei are tiny because the nuclear forc ...
... • When two nucleons are just a few nucleon diameters apart, the nuclear force they exert on each other is nearly zero (small force vectors). • This means that if nucleons are to be held together by the strong force, they must be held in a very small volume. • Nuclei are tiny because the nuclear forc ...
39 The Atomic Nucleus and Radioactivity
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. They will lose their energy after a large number of glancing collisions with atomic electrons. Beta particles slow down until they bec ...
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. They will lose their energy after a large number of glancing collisions with atomic electrons. Beta particles slow down until they bec ...
transmutation of nuclides
... nuclide, because this nuclide no longer exists in the planet Earth. Studies of radioactive decays led to theories of nuclear stability and nuclear structure. Some of these theories will be examined as we take a closer look at atomic nuclei. Concepts such as energy states of nucleons, angular momentu ...
... nuclide, because this nuclide no longer exists in the planet Earth. Studies of radioactive decays led to theories of nuclear stability and nuclear structure. Some of these theories will be examined as we take a closer look at atomic nuclei. Concepts such as energy states of nucleons, angular momentu ...
Radioactive Decays – transmutations of nuclides
... 3. A plutonium bomb used 500 kg of 239Pu in 1959. This bomb is sitting in a warehouse silo in the US desert. How much 239Pu is left in the bomb today? (The overall half-life for 239Pu is 24400 y, and it has several decay modes) 4. A hydrogen bomb used 5 kg of 3T in 1959. This bomb is sitting in a wa ...
... 3. A plutonium bomb used 500 kg of 239Pu in 1959. This bomb is sitting in a warehouse silo in the US desert. How much 239Pu is left in the bomb today? (The overall half-life for 239Pu is 24400 y, and it has several decay modes) 4. A hydrogen bomb used 5 kg of 3T in 1959. This bomb is sitting in a wa ...
Slide 1
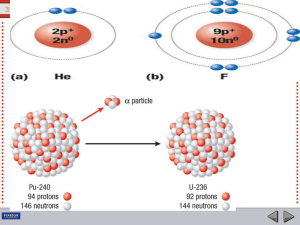
... When an atom ejects an alpha particle, the mass number of the resulting atom decreases by 4, and the atomic number by 2. The resulting atom belongs to an element two spaces back in the periodic table. When an atom ejects a beta particle from its nucleus, it loses no nucleons, its atomic number incre ...
... When an atom ejects an alpha particle, the mass number of the resulting atom decreases by 4, and the atomic number by 2. The resulting atom belongs to an element two spaces back in the periodic table. When an atom ejects a beta particle from its nucleus, it loses no nucleons, its atomic number incre ...
eXtremely Fast Tr
... While low-energy cosmic rays such as the solar wind cause ionization in the upper atmosphere, muons cause most of the ionization in the lower atmosphere. When a muon ionizes a gas molecule, it strips away an electron, making that molecule into a positive ion. The electron is soon captured, either by ...
... While low-energy cosmic rays such as the solar wind cause ionization in the upper atmosphere, muons cause most of the ionization in the lower atmosphere. When a muon ionizes a gas molecule, it strips away an electron, making that molecule into a positive ion. The electron is soon captured, either by ...
Estimation of the fluence of high‐energy electron bursts produced by
... The avalanche is initiated by 10 energetic electrons at the bottom of the high‐field region, which might correspond to the injection of energetic electrons by a lightning leader. The simulation calculates but does not plot the X rays produced by bremsstrahlung emission. For a realistic thundercloud ...
... The avalanche is initiated by 10 energetic electrons at the bottom of the high‐field region, which might correspond to the injection of energetic electrons by a lightning leader. The simulation calculates but does not plot the X rays produced by bremsstrahlung emission. For a realistic thundercloud ...
Topic 7_2__Radioactive decay
... What this means is that an unstable nucleus may spontaneously decay into another nucleus (which may or may not be stable). Given many identical unstable nuclides, which precise ones will decay in any particular time is impossible to predict. In other words, the decay process is random. But rando ...
... What this means is that an unstable nucleus may spontaneously decay into another nucleus (which may or may not be stable). Given many identical unstable nuclides, which precise ones will decay in any particular time is impossible to predict. In other words, the decay process is random. But rando ...
PRINCIPLES OF RADIATION DETECTION AND QUANTIFICATION
... living tissue is emitted as the unstable atoms (radionuclides) change ('decay') spontaneously to become different kinds of atoms. The principal kinds of ionizing radiation are: Alpha particles These are helium nuclei consisting of two protons and two neutrons and are emitted from naturally-occurring ...
... living tissue is emitted as the unstable atoms (radionuclides) change ('decay') spontaneously to become different kinds of atoms. The principal kinds of ionizing radiation are: Alpha particles These are helium nuclei consisting of two protons and two neutrons and are emitted from naturally-occurring ...
Beta decay is a type of radioactive decay in which a beta
... charge, Z. Therefore the set of allnuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. A beta-stable nucleus may undergo other kinds of radioactive decay (for example, alpha decay). In nature, most isotopes are beta-stable, but there exist a f ...
... charge, Z. Therefore the set of allnuclides with the same A can be introduced; these isobaric nuclides may turn into each other via beta decay. A beta-stable nucleus may undergo other kinds of radioactive decay (for example, alpha decay). In nature, most isotopes are beta-stable, but there exist a f ...
radioactivity and radioactive decay - rct study guide
... electrons existed, then when they encountered an ordinary negative electron, the Coulomb force would cause the two particles to accelerate toward each other. They would collide and then the two equal but opposite charges would mutually cancel. This would leave two neutral electrons. Actually, a posi ...
... electrons existed, then when they encountered an ordinary negative electron, the Coulomb force would cause the two particles to accelerate toward each other. They would collide and then the two equal but opposite charges would mutually cancel. This would leave two neutral electrons. Actually, a posi ...
Magnetic and Electric Deviation of the Easily Absorbed Rays
... that the atoms of these substances sire made up, in part at least, of rapidly rotating or oscillating systems of heavy charged bodies large compared with the electron. The sudden escape of these masses from their orbit may be due either to the action of internal forces or external forces of which we ...
... that the atoms of these substances sire made up, in part at least, of rapidly rotating or oscillating systems of heavy charged bodies large compared with the electron. The sudden escape of these masses from their orbit may be due either to the action of internal forces or external forces of which we ...
THE ATOMIC NUCLEUS AND RADIOACTIVITY
... energy equivalence. Particles decay spontaneously only when their combined products have less mass after decay than before. The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). So when a neutron decays, there is less mass after decay than be ...
... energy equivalence. Particles decay spontaneously only when their combined products have less mass after decay than before. The mass of a neutron is slightly greater than the total mass of a proton plus electron (and the antineutrino). So when a neutron decays, there is less mass after decay than be ...
PART I TORT LIABILITY AND RADIATION INJURIES
... in its most common form, has a weight, or mass, of approximately one. The heaviest naturally occurring element is uranium, with an atomic weight (mass number) of 238, derived from 92 protons (atomic number 92) and 146 neutrons in its nucleus. Between these two naturally occuring elements 90 others a ...
... in its most common form, has a weight, or mass, of approximately one. The heaviest naturally occurring element is uranium, with an atomic weight (mass number) of 238, derived from 92 protons (atomic number 92) and 146 neutrons in its nucleus. Between these two naturally occuring elements 90 others a ...
Critical Thinking Questions 2
... (b) β decay: a neutron in the nucleus is converted into a proton and an electron. The electron is ejected from the nucleus. (c) Positron or β+ emission: a proton in the nucleus is converted into a neutron and a positron. The positron is ejected from the nucleus. (d) Electron capture: the nucleus cap ...
... (b) β decay: a neutron in the nucleus is converted into a proton and an electron. The electron is ejected from the nucleus. (c) Positron or β+ emission: a proton in the nucleus is converted into a neutron and a positron. The positron is ejected from the nucleus. (d) Electron capture: the nucleus cap ...
4 Radioactive Elements
... chemical reaction will not convert one element into a different element. Such a change happens only during nuclear reactions (NOO klee ur)—reactions involving the particles in the nucleus of an atom. Remember that atoms with the same number of protons and different numbers of neutrons are called iso ...
... chemical reaction will not convert one element into a different element. Such a change happens only during nuclear reactions (NOO klee ur)—reactions involving the particles in the nucleus of an atom. Remember that atoms with the same number of protons and different numbers of neutrons are called iso ...
University of Victoria Radiation Safety Refresher Course
... • Very high-energy ionizing radiation. • Have no mass and no electrical charge. • Occurs when the nucleus of a radioactive atom has too much energy. Often follows the emission of a beta particle. • Highly penetrating – requires lead or concrete shielding. • Causes severe damage to internal orga ...
... • Very high-energy ionizing radiation. • Have no mass and no electrical charge. • Occurs when the nucleus of a radioactive atom has too much energy. Often follows the emission of a beta particle. • Highly penetrating – requires lead or concrete shielding. • Causes severe damage to internal orga ...
Pdf - Text of NPTEL IIT Video Lectures
... having the same number of protons, but different number of neutrons. So, we will discuss this in detail a little later isotopes of an element have seem characteristic atomic number, so that means it have same number of protons in their atomic nuclei, but they have different mass number. So, these at ...
... having the same number of protons, but different number of neutrons. So, we will discuss this in detail a little later isotopes of an element have seem characteristic atomic number, so that means it have same number of protons in their atomic nuclei, but they have different mass number. So, these at ...
Physics and Chemistry 1501 – Nuclear Science Part I VO Atomic
... we’re going to keep things simple and omit it. Beta particles have higher penetrating power than alpha. They go right through paper and are stopped by a sheet of aluminum. The third type of radioactive emission is the gamma ray. We use the Greek letter gamma as its symbol. Since gamma rays are energ ...
... we’re going to keep things simple and omit it. Beta particles have higher penetrating power than alpha. They go right through paper and are stopped by a sheet of aluminum. The third type of radioactive emission is the gamma ray. We use the Greek letter gamma as its symbol. Since gamma rays are energ ...
Nuclear Physics - Assam Valley School
... (ii) How is it possible for an element to decay into another element of higher atomic number ? (iii) Is it possible for hydrogen atom isotope to emit alpha particle ? Explain. Ans. (i) γ-radiations are electromagnetic in nature and as such obey the laws of reflection, refraction and are not affected ...
... (ii) How is it possible for an element to decay into another element of higher atomic number ? (iii) Is it possible for hydrogen atom isotope to emit alpha particle ? Explain. Ans. (i) γ-radiations are electromagnetic in nature and as such obey the laws of reflection, refraction and are not affected ...
AP Revision Guide Ch 18
... 4. X-radiation which consists of high-energy photons emitted when fast-moving electrons are stopped in an x-ray tube. 5. Mesons from cosmic rays striking the atmosphere. Alpha radiation The alpha particles from any one type of decay all have the same energy, typically a few MeV. Being relatively mas ...
... 4. X-radiation which consists of high-energy photons emitted when fast-moving electrons are stopped in an x-ray tube. 5. Mesons from cosmic rays striking the atmosphere. Alpha radiation The alpha particles from any one type of decay all have the same energy, typically a few MeV. Being relatively mas ...
Radiation Safety - 7
... exposed to radiation record Photons (X & d Rays) in the 5 keV / 40 MeV range & Beta Particles in the 150 keV / 10 MeV range. During analysis, the Al2O3 is stimulated with selected frequencies of laser light, which cause it to become luminescent in proportion to the amount of radiation exposure recei ...
... exposed to radiation record Photons (X & d Rays) in the 5 keV / 40 MeV range & Beta Particles in the 150 keV / 10 MeV range. During analysis, the Al2O3 is stimulated with selected frequencies of laser light, which cause it to become luminescent in proportion to the amount of radiation exposure recei ...
Radioactive Decay
... Radioactive Decay Example: Assuming a half-life of _________, how many years will be needed for the decay of ________ of a given amount of radium-226? Amount remaining = 1/16 = 0.0625 = (½)4 = ____________ Years needed for decay of 15/16 = (1599 years) (__) = ___________ ...
... Radioactive Decay Example: Assuming a half-life of _________, how many years will be needed for the decay of ________ of a given amount of radium-226? Amount remaining = 1/16 = 0.0625 = (½)4 = ____________ Years needed for decay of 15/16 = (1599 years) (__) = ___________ ...
Gamma ray
Gamma radiation, also known as gamma rays, and denoted by the Greek letter γ, refers to electromagnetic radiation of an extremely high frequency and therefore consists of high-energy photons. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay of atomic nuclei as they transition from a high energy state to a lower state known as gamma decay, but may also be produced by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named ""gamma rays"" by Ernest Rutherford in 1903.Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes, and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes. Additionally, gamma rays are produced by a number of astronomical processes in which very high-energy electrons are produced, that in turn cause secondary gamma rays via bremsstrahlung, inverse Compton scattering, and synchrotron radiation. However, a large fraction of such astronomical gamma rays are screened by Earth's atmosphere and can only be detected by spacecraft.Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (10−12 meter), which is less than the diameter of an atom. However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes. Electromagnetic radiation from radioactive decay of atomic nuclei is referred to as ""gamma rays"" no matter its energy, so that there is no lower limit to gamma energy derived from radioactive decay. This radiation commonly has energy of a few hundred keV, and almost always less than 10 MeV. In astronomy, gamma rays are defined by their energy, and no production process needs to be specified. The energies of gamma rays from astronomical sources range to over 10 TeV, an energy far too large to result from radioactive decay. A notable example is extremely powerful bursts of high-energy radiation referred to as long duration gamma-ray bursts, of energies higher than can be produced by radioactive decay. These bursts of gamma rays, thought to be due to the collapse of stars called hypernovae, are the most powerful events so far discovered in the cosmos.