Radioactive Decay
... Atoms are likely to undergo β+ decay if they have too many protons and not enough neutrons to achieve a stable neutron/proton ratio. Note that β+ decay and electron capture produce the same products. Electron capture can sometimes (but not often) occur without β+ decay, but β+ decay is always accomp ...
... Atoms are likely to undergo β+ decay if they have too many protons and not enough neutrons to achieve a stable neutron/proton ratio. Note that β+ decay and electron capture produce the same products. Electron capture can sometimes (but not often) occur without β+ decay, but β+ decay is always accomp ...
Objectives for Nuclear Chemistry
... mass. The calculated mass is derived from adding the masses of protons and neutrons that are present. The actual mass is less than this. The difference is due to the fact that the formation of a nucleus releases a tremendous amount of energy. Objective 11 The energy released in the formation of an a ...
... mass. The calculated mass is derived from adding the masses of protons and neutrons that are present. The actual mass is less than this. The difference is due to the fact that the formation of a nucleus releases a tremendous amount of energy. Objective 11 The energy released in the formation of an a ...
Unit 2: The Atom
... •Alpha decay is how elements greater than atomic #83 try to become stable. •They will emit an alpha particle (2 neutrons and 2 protons) to try to become stable. •Alpha reactions will always have He on the right side! •To balance: write the upper and lower equations! ...
... •Alpha decay is how elements greater than atomic #83 try to become stable. •They will emit an alpha particle (2 neutrons and 2 protons) to try to become stable. •Alpha reactions will always have He on the right side! •To balance: write the upper and lower equations! ...
The Mössbauer Effect in 57Fe
... shift caused by the recoil of nuclei is also known from atomic physics, but there the shift is much smaller since the transition energies and therefore the recoil energies are much lower than in the case of nuclear physics. In 1958, Rudolph Mössbauer discovered that some of the nuclei in the crysta ...
... shift caused by the recoil of nuclei is also known from atomic physics, but there the shift is much smaller since the transition energies and therefore the recoil energies are much lower than in the case of nuclear physics. In 1958, Rudolph Mössbauer discovered that some of the nuclei in the crysta ...
Radiation_What Is It
... of the proton rich atom. This positive electron is known as a positron. An additional particle, a neutrino, is also emitted from the nucleus. Neutrinos are very small particles with no electric charge. They have little or no mass and participate in weak interactions. ...
... of the proton rich atom. This positive electron is known as a positron. An additional particle, a neutrino, is also emitted from the nucleus. Neutrinos are very small particles with no electric charge. They have little or no mass and participate in weak interactions. ...
IONIZING RADIATION AND RADIONUCLIDS AS THE SOURSES …
... • Ionizing radiation occurs in two forms – particles or waves. • Alpha particles is not external hazard and can bee shielded against by clothing. Internal deposition of alpha particles is of importance on a long-term basis in terms of causing radiation injury. • Beta irradiation causes damage to the ...
... • Ionizing radiation occurs in two forms – particles or waves. • Alpha particles is not external hazard and can bee shielded against by clothing. Internal deposition of alpha particles is of importance on a long-term basis in terms of causing radiation injury. • Beta irradiation causes damage to the ...
do physics online from quanta to quarks radioactivity
... The need to account for the energy distribution of electrons emitted in beta decay (figure 1) and to satisfy the laws of conservation of energy, linear and angular momentum, Austrian physicist Wolfgang Pauli in 1930 proposed that a neutral particle was emitted along with the particle. This partic ...
... The need to account for the energy distribution of electrons emitted in beta decay (figure 1) and to satisfy the laws of conservation of energy, linear and angular momentum, Austrian physicist Wolfgang Pauli in 1930 proposed that a neutral particle was emitted along with the particle. This partic ...
Basics of nuclear physics
... collides with electron, both disappear, creating two gamma photons of 511 keV energy, going in opposite direction Only for photons with high energies interacting with high-atomic-number elements ...
... collides with electron, both disappear, creating two gamma photons of 511 keV energy, going in opposite direction Only for photons with high energies interacting with high-atomic-number elements ...
Nuclear Notation
... Gamma Decay Gamma rays are not charged particles like α and β particles. Gamma rays are electromagnetic radiation with high frequency. When a nucleus undergoes a transition from higher to lower energy Level ,they are equivalent to light quanta and X rays ,their energies much greater and the half li ...
... Gamma Decay Gamma rays are not charged particles like α and β particles. Gamma rays are electromagnetic radiation with high frequency. When a nucleus undergoes a transition from higher to lower energy Level ,they are equivalent to light quanta and X rays ,their energies much greater and the half li ...
Chapter 25
... man-made. Most of them only exist for a second or less and then they decay into something else. Many of them were made by Dr. Seaborg and his team of researchers. ...
... man-made. Most of them only exist for a second or less and then they decay into something else. Many of them were made by Dr. Seaborg and his team of researchers. ...
Radioactivityunit6
... • Gamma rays = (o) nuetral, electromagnetic waves (light waves) Rutherford figured out the charged by placing an electric and magnetic field around the radioactive element to see where the particles curved. ...
... • Gamma rays = (o) nuetral, electromagnetic waves (light waves) Rutherford figured out the charged by placing an electric and magnetic field around the radioactive element to see where the particles curved. ...
radiation!!! - Mr Schmitt
... When these nuclei lose energy and break apart, decay occurs ▪ Radioactive decay releases energy from the nucleus as radiation ▪ Radioactive atoms release energy until they become stable, often ending up as different atoms ▪ For example: uranium-238 (parent nucleus) decays in several stages until i ...
... When these nuclei lose energy and break apart, decay occurs ▪ Radioactive decay releases energy from the nucleus as radiation ▪ Radioactive atoms release energy until they become stable, often ending up as different atoms ▪ For example: uranium-238 (parent nucleus) decays in several stages until i ...
Chapter 25 Nuclear Chemistry
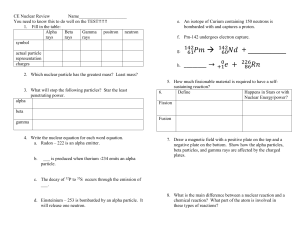
... Ex. Write the radioactive decay for Plutonium -239 and Uranium-235 below (both nuclei emit alpha particles - table N) ...
... Ex. Write the radioactive decay for Plutonium -239 and Uranium-235 below (both nuclei emit alpha particles - table N) ...
Chapter 28 for Chem
... of energy. Paper can stop alpha particles. Beta particles have more energy than alpha, but less than gamma. Aluminum foil or a thin piece of wood can stop beta particles. Gamma particles have the most energy by far. Several meters of concrete will stop them as will several centimeters of lead. T ...
... of energy. Paper can stop alpha particles. Beta particles have more energy than alpha, but less than gamma. Aluminum foil or a thin piece of wood can stop beta particles. Gamma particles have the most energy by far. Several meters of concrete will stop them as will several centimeters of lead. T ...
Radiation and Radioactive Decay
... we notice that the nuclei near the middle of the period table are held together more strongly than other nuclei. Why is it that the middle elements have the highest binding energies? The nucleus is the battleground between the strong nuclear (only active at a distance of a small nucleus) and the el ...
... we notice that the nuclei near the middle of the period table are held together more strongly than other nuclei. Why is it that the middle elements have the highest binding energies? The nucleus is the battleground between the strong nuclear (only active at a distance of a small nucleus) and the el ...
Chapter 29: Nuclear Physics
... Alpha, beta, and gamma radiation penetrates to different depths in biological materials. •Alpha rays are stopped by a few cm of air or about 0.02 mm of aluminum. •Beta-minus can penetrate a few cm into biological tissue. •Gamma ray absorption is based on probability so they can penetrate to varying ...
... Alpha, beta, and gamma radiation penetrates to different depths in biological materials. •Alpha rays are stopped by a few cm of air or about 0.02 mm of aluminum. •Beta-minus can penetrate a few cm into biological tissue. •Gamma ray absorption is based on probability so they can penetrate to varying ...
Problem 1 - Department of Physics and Astronomy : University of
... Whew! Welcome to the end of your introductory physics sequence! I hope you will find some of what you have learned about useful through the years. You’ve been great sports. As for grades: I must make a research trip from Thursday-Sunday. During that time the finals will be graded. On Monday, May 12 ...
... Whew! Welcome to the end of your introductory physics sequence! I hope you will find some of what you have learned about useful through the years. You’ve been great sports. As for grades: I must make a research trip from Thursday-Sunday. During that time the finals will be graded. On Monday, May 12 ...
Atomic nuclei: radioactivity and types of radiation
... low penetration power. Penetration power describes how easily the particles can pass through another material. Because alpha particles have a low penetration power, it means that even something as thin as a piece of paper, or the outside layer of the human skin, will absorb these particles so that t ...
... low penetration power. Penetration power describes how easily the particles can pass through another material. Because alpha particles have a low penetration power, it means that even something as thin as a piece of paper, or the outside layer of the human skin, will absorb these particles so that t ...
Nuclear Chemistry - Mrs. Carlyle`s Classroom
... Atomic nuclei are made of protons and neutrons, which are collectively called nucleons Nuclear radiation – particles or electromagnetic radiation emitted from the nucleus during radioactive decay Radioactive nuclide – an unstable nucleus that undergoes radioactive decay Nuclear reaction – a r ...
... Atomic nuclei are made of protons and neutrons, which are collectively called nucleons Nuclear radiation – particles or electromagnetic radiation emitted from the nucleus during radioactive decay Radioactive nuclide – an unstable nucleus that undergoes radioactive decay Nuclear reaction – a r ...
Chapter 16 Notes - Mr. Julien`s Homepage
... 2. Paper, clothing, and skin will protect you from alpha particles. 3. Beta particles have a very small mass and move much faster and farther than alpha particles, traveling as much as several meters through air. 4. Beta particles can penetrate as far as 4-5 mm into the body, burning the surface of ...
... 2. Paper, clothing, and skin will protect you from alpha particles. 3. Beta particles have a very small mass and move much faster and farther than alpha particles, traveling as much as several meters through air. 4. Beta particles can penetrate as far as 4-5 mm into the body, burning the surface of ...
1 0 +1 0 - davis.k12.ut.us
... nucleus, releasing lots of energy as the nucleus splits or fuses. Chemical reactions happen with the electrons, bonds are being broken or formed. ...
... nucleus, releasing lots of energy as the nucleus splits or fuses. Chemical reactions happen with the electrons, bonds are being broken or formed. ...
Gamma ray
Gamma radiation, also known as gamma rays, and denoted by the Greek letter γ, refers to electromagnetic radiation of an extremely high frequency and therefore consists of high-energy photons. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay of atomic nuclei as they transition from a high energy state to a lower state known as gamma decay, but may also be produced by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named ""gamma rays"" by Ernest Rutherford in 1903.Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes, and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes. Additionally, gamma rays are produced by a number of astronomical processes in which very high-energy electrons are produced, that in turn cause secondary gamma rays via bremsstrahlung, inverse Compton scattering, and synchrotron radiation. However, a large fraction of such astronomical gamma rays are screened by Earth's atmosphere and can only be detected by spacecraft.Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (10−12 meter), which is less than the diameter of an atom. However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes. Electromagnetic radiation from radioactive decay of atomic nuclei is referred to as ""gamma rays"" no matter its energy, so that there is no lower limit to gamma energy derived from radioactive decay. This radiation commonly has energy of a few hundred keV, and almost always less than 10 MeV. In astronomy, gamma rays are defined by their energy, and no production process needs to be specified. The energies of gamma rays from astronomical sources range to over 10 TeV, an energy far too large to result from radioactive decay. A notable example is extremely powerful bursts of high-energy radiation referred to as long duration gamma-ray bursts, of energies higher than can be produced by radioactive decay. These bursts of gamma rays, thought to be due to the collapse of stars called hypernovae, are the most powerful events so far discovered in the cosmos.