Ch. 21.1 Nuclear Radiation
... – These changes are always accompanied by the emission of large amounts of energy. – Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. – Nuclear reactions of given radioisotope cannot be slowed down, speeded up, or stopp ...
... – These changes are always accompanied by the emission of large amounts of energy. – Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. – Nuclear reactions of given radioisotope cannot be slowed down, speeded up, or stopp ...
Chp 7.1 Atomic Theory and Radioactive Decay
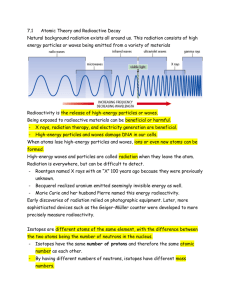
... • Natural background radiation exists all around us. • This radiation consists of high energy particles or waves being emitted from a variety of materials. ...
... • Natural background radiation exists all around us. • This radiation consists of high energy particles or waves being emitted from a variety of materials. ...
Radioactivity
... • The discovery of radioactivity by Becquerel and the Curies showed that one of Dalton’s ideas, that matter is indestructible and indivisible, is not ...
... • The discovery of radioactivity by Becquerel and the Curies showed that one of Dalton’s ideas, that matter is indestructible and indivisible, is not ...
Radioactivity2015
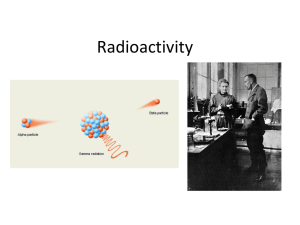
... SUMMARY of alpha, beta, gamma Alpha particles • An alpha particle is simply a helium nuclei (He) which is ejected with high energy from an unstable nucleus • This particle, which consists of two protons and two neutrons, has a net positive charge. • Although emitted with high energy, alpha particle ...
... SUMMARY of alpha, beta, gamma Alpha particles • An alpha particle is simply a helium nuclei (He) which is ejected with high energy from an unstable nucleus • This particle, which consists of two protons and two neutrons, has a net positive charge. • Although emitted with high energy, alpha particle ...
Student 5
... What conclusions were made about the atom from Rutherford’s gold leaf experiment? Rutherford fired a beam of alpha particles at thin gold foil. The alpha particles were from a radioactive source, in an evacuated container. A scintillation detector then rotated around the container was used to pick u ...
... What conclusions were made about the atom from Rutherford’s gold leaf experiment? Rutherford fired a beam of alpha particles at thin gold foil. The alpha particles were from a radioactive source, in an evacuated container. A scintillation detector then rotated around the container was used to pick u ...
File
... photon is emitted (light) • Gamma rays have no mass, no p+ and no no so they do not alter atomic mass or atomic number. • It is PURE ENERGY which explains the large penetrating power of rays. • They are often emitted along with alpha or beta particles 230 Th 226 Ra + 4 He + ...
... photon is emitted (light) • Gamma rays have no mass, no p+ and no no so they do not alter atomic mass or atomic number. • It is PURE ENERGY which explains the large penetrating power of rays. • They are often emitted along with alpha or beta particles 230 Th 226 Ra + 4 He + ...
Notes - Science With Horne
... photon is emitted (light) • Gamma rays have no mass, no p+ and no no so they do not alter atomic mass or atomic number. • It is PURE ENERGY which explains the large penetrating power of rays. • They are often emitted along with alpha or beta particles 230 Th 226 Ra + 4 He + ...
... photon is emitted (light) • Gamma rays have no mass, no p+ and no no so they do not alter atomic mass or atomic number. • It is PURE ENERGY which explains the large penetrating power of rays. • They are often emitted along with alpha or beta particles 230 Th 226 Ra + 4 He + ...
Isotope Half-Life Radiation Emitted
... C. Gamma Decay • Emission of Gamma radiation-- g • Gamma rays are electromagnetic waves or energy • They have no mass. • Gamma radiation has no charge. • Most Penetrating, can be stopped by 1m thick concrete or a several cm thick sheet of lead. ...
... C. Gamma Decay • Emission of Gamma radiation-- g • Gamma rays are electromagnetic waves or energy • They have no mass. • Gamma radiation has no charge. • Most Penetrating, can be stopped by 1m thick concrete or a several cm thick sheet of lead. ...
SCIENCE 10: (7.1) ATOMIC THEORY, ISOTOPES
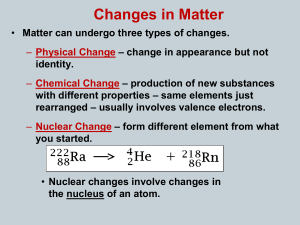
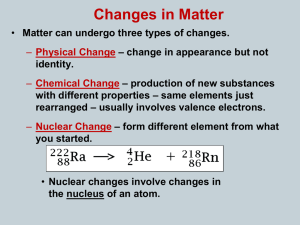
... ***Note: The equation is balanced! (The sum of the atomic numbers and the sum of the mass numbers are equal on both sides of the arrow. Also the product nucleus has an atomic number that is greater, making it an atom of the next higher element and a mass number that equal to the reactant because the ...
... ***Note: The equation is balanced! (The sum of the atomic numbers and the sum of the mass numbers are equal on both sides of the arrow. Also the product nucleus has an atomic number that is greater, making it an atom of the next higher element and a mass number that equal to the reactant because the ...
Nuclear Chemistry - Northwest ISD Moodle
... Mass number does not change Add 1 to atomic number Identify new element from atomic number Add beta particle ...
... Mass number does not change Add 1 to atomic number Identify new element from atomic number Add beta particle ...
AP Chem
... 25. Read about Japanese nuclear power plant accident. Write facts about the accident and current effects. ( ½ page) ...
... 25. Read about Japanese nuclear power plant accident. Write facts about the accident and current effects. ( ½ page) ...
(or radioactive isotopes).
... • Gamma rays are used to kill bacteria, mould and insects in food. They are also used to kill bacteria on hospital equipment, dressings and bandages. • This is useful particularly on packaged food or on plastic items which would be damaged by heat sterilisation. • There are arguments for using cobal ...
... • Gamma rays are used to kill bacteria, mould and insects in food. They are also used to kill bacteria on hospital equipment, dressings and bandages. • This is useful particularly on packaged food or on plastic items which would be damaged by heat sterilisation. • There are arguments for using cobal ...
Radioactivity Reading Assignment Name: Chemistry Date: Hour
... away some energy. Gamma rays (ã) are electromagnetic waves, rather like X-rays and radio waves. Thus gamma rays have no mass and no charge. á-particles and â-particles pull electrons off atoms as they pass (we say they ionize the atoms), but ã rays don’t. This means that they do not lose much energy ...
... away some energy. Gamma rays (ã) are electromagnetic waves, rather like X-rays and radio waves. Thus gamma rays have no mass and no charge. á-particles and â-particles pull electrons off atoms as they pass (we say they ionize the atoms), but ã rays don’t. This means that they do not lose much energy ...
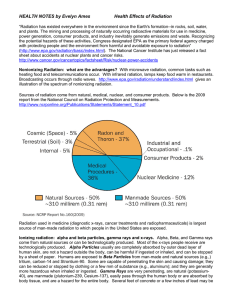
Health Effects of Radiation
... bone and teeth, causing damage to teeth and increasing the risk of bone cancer. How can gamma particles affect people’s health? “Because of the gamma ray's penetrating power and ability to travel great distances, it is considered the primary hazard to the general population during most radiological ...
... bone and teeth, causing damage to teeth and increasing the risk of bone cancer. How can gamma particles affect people’s health? “Because of the gamma ray's penetrating power and ability to travel great distances, it is considered the primary hazard to the general population during most radiological ...
Unit 14 Notes - shscience.net
... protons, 2 neutrons 42He nucleus Beta Particle (β-) Electron emitted from the nucleus 0-1e Positron Particle (β+) Mass of an electron but positive charge 0+1e Gamma Radiation (γ) High energy radiation (higher than x-ray) No mass and no charge ...
... protons, 2 neutrons 42He nucleus Beta Particle (β-) Electron emitted from the nucleus 0-1e Positron Particle (β+) Mass of an electron but positive charge 0+1e Gamma Radiation (γ) High energy radiation (higher than x-ray) No mass and no charge ...
AP Exam Questions: Nuclear
... 43. The atomic mass of copper is 63.55. Given that there are only two naturally occurring isotopes of copper, ...
... 43. The atomic mass of copper is 63.55. Given that there are only two naturally occurring isotopes of copper, ...
File - Ms M - EARL MARRIOTT SECONDARY
... Early discoveries of radiation relied on photographic equipment. Later, more sophisticated devices such as the Geiger-Müller counter were developed to more precisely measure radioactivity. Isotopes are different atoms of the same element, with the difference between the two atoms being the number of ...
... Early discoveries of radiation relied on photographic equipment. Later, more sophisticated devices such as the Geiger-Müller counter were developed to more precisely measure radioactivity. Isotopes are different atoms of the same element, with the difference between the two atoms being the number of ...
30.1 Radioactivity The atom is the smallest unit of achemical
... a proton decays into a neutron it has the same charge as electron but negative charge • it has the same mass as electron • it can penetrate with few meters in air. 2 or 3 cm of wood are enough to protect oneself. 3- Gamma decay (γ) ...
... a proton decays into a neutron it has the same charge as electron but negative charge • it has the same mass as electron • it can penetrate with few meters in air. 2 or 3 cm of wood are enough to protect oneself. 3- Gamma decay (γ) ...
solutions - Physicsland
... nearly 2000 times the mass of an electron). Hence it bends very little compared to the much less massive beta particles (electrons). Gamma rays carry no electric charge and so are not affected by an electric field. 9. Gamma radiation produces not only the least change in mass and atomic numbers, but ...
... nearly 2000 times the mass of an electron). Hence it bends very little compared to the much less massive beta particles (electrons). Gamma rays carry no electric charge and so are not affected by an electric field. 9. Gamma radiation produces not only the least change in mass and atomic numbers, but ...
Radioactivity
... • 7.4 understand that alpha and beta particles and gamma rays are ionising radiations emitted from unstable nuclei in a random process • 7.5 describe the nature of alpha and beta particles and gamma rays and recall that they may be distinguished in terms of penetrating power • 7.6 describe the effec ...
... • 7.4 understand that alpha and beta particles and gamma rays are ionising radiations emitted from unstable nuclei in a random process • 7.5 describe the nature of alpha and beta particles and gamma rays and recall that they may be distinguished in terms of penetrating power • 7.6 describe the effec ...
Average Atomic Mass
... • The third common type of radiation is gamma radiation or gamma rays. • Gamma rays are high-energy radiation that possess no mass and have no charge. • Gamma rays are denoted by the symbol 00γ. • Gamma rays usually accompany alpha and beta radiation and account for most of the energy lost during th ...
... • The third common type of radiation is gamma radiation or gamma rays. • Gamma rays are high-energy radiation that possess no mass and have no charge. • Gamma rays are denoted by the symbol 00γ. • Gamma rays usually accompany alpha and beta radiation and account for most of the energy lost during th ...
5.7 Nuclear Radiation
... – These changes are always accompanied by the emission of large amounts of energy. – Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. – Nuclear reactions of given radioisotope cannot be slowed down, speeded up, or stopp ...
... – These changes are always accompanied by the emission of large amounts of energy. – Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. – Nuclear reactions of given radioisotope cannot be slowed down, speeded up, or stopp ...
Nuclear Chemistry
... A Beta particle is an electron created and emitted when a neutron is transformed* into a proton and an electron during radioactive decay. This action adds a proton and thus changes the identity of the atom. The mass number stays the same. ...
... A Beta particle is an electron created and emitted when a neutron is transformed* into a proton and an electron during radioactive decay. This action adds a proton and thus changes the identity of the atom. The mass number stays the same. ...
Nuclear Decay
... – An electron emitted from a decaying neutron in the nucleus – Does not have an atomic mass – Atomic number of -1 ...
... – An electron emitted from a decaying neutron in the nucleus – Does not have an atomic mass – Atomic number of -1 ...
2005 Nuclear FRQs - AP Chemistry Olympics
... Teachers may reproduce this publication, in whole or in part, in limited print quantities for non-commercial, face-to-face teaching purposes. This permission does not apply to any third-party copyrights contained within this publication. Advanced Placement Examination in Chemistry. Questions copyrig ...
... Teachers may reproduce this publication, in whole or in part, in limited print quantities for non-commercial, face-to-face teaching purposes. This permission does not apply to any third-party copyrights contained within this publication. Advanced Placement Examination in Chemistry. Questions copyrig ...
Gamma ray
Gamma radiation, also known as gamma rays, and denoted by the Greek letter γ, refers to electromagnetic radiation of an extremely high frequency and therefore consists of high-energy photons. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay of atomic nuclei as they transition from a high energy state to a lower state known as gamma decay, but may also be produced by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named ""gamma rays"" by Ernest Rutherford in 1903.Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes, and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes. Additionally, gamma rays are produced by a number of astronomical processes in which very high-energy electrons are produced, that in turn cause secondary gamma rays via bremsstrahlung, inverse Compton scattering, and synchrotron radiation. However, a large fraction of such astronomical gamma rays are screened by Earth's atmosphere and can only be detected by spacecraft.Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (10−12 meter), which is less than the diameter of an atom. However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes. Electromagnetic radiation from radioactive decay of atomic nuclei is referred to as ""gamma rays"" no matter its energy, so that there is no lower limit to gamma energy derived from radioactive decay. This radiation commonly has energy of a few hundred keV, and almost always less than 10 MeV. In astronomy, gamma rays are defined by their energy, and no production process needs to be specified. The energies of gamma rays from astronomical sources range to over 10 TeV, an energy far too large to result from radioactive decay. A notable example is extremely powerful bursts of high-energy radiation referred to as long duration gamma-ray bursts, of energies higher than can be produced by radioactive decay. These bursts of gamma rays, thought to be due to the collapse of stars called hypernovae, are the most powerful events so far discovered in the cosmos.