nuclear test 2006
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...
... Carbon Dating is a technique used to determine the age of organic material. The activity (rate of decay) of 14C atoms in the sample is measured. 14C has a half life of 5730 years. a) Explain how the activity of 14C in an organic sample can be used to determine its age. ...
Radioactivity Notes Day 1 and 2 Apr 23 and Apr 24
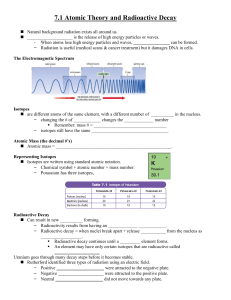
... Isotopes are different atoms of the same element, with the difference between the two atoms being the number of neutrons in the nucleus. o Eg. Carbon-12, Carbon-13, Carbon-14 o By having different numbers of neutrons, isotopes have different mass numbers. Isotopes of an element have the same sym ...
... Isotopes are different atoms of the same element, with the difference between the two atoms being the number of neutrons in the nucleus. o Eg. Carbon-12, Carbon-13, Carbon-14 o By having different numbers of neutrons, isotopes have different mass numbers. Isotopes of an element have the same sym ...
NUCLEAR CHEMISTRY
... A. Nuclear Fission 1. A very heavy nucleus splits into more stable nuclei of intermediate mass 2. The mass of the products is less than the mass of the reactants. Missing mass is converted to energy a. Small amounts of missing mass are converted to HUGE amounts of energy (E = mc2) ...
... A. Nuclear Fission 1. A very heavy nucleus splits into more stable nuclei of intermediate mass 2. The mass of the products is less than the mass of the reactants. Missing mass is converted to energy a. Small amounts of missing mass are converted to HUGE amounts of energy (E = mc2) ...
Chemistry: Matter and Change
... • Gamma rays (short wavelength) are photons, which are high-energy • Gamma rays have no mass or charge so the emission of gamma rays does not change the atomic number or mass number of a nucleus. • Gamma rays almost always accompany alpha and beta radiation. • X rays are a form of high-energy electr ...
... • Gamma rays (short wavelength) are photons, which are high-energy • Gamma rays have no mass or charge so the emission of gamma rays does not change the atomic number or mass number of a nucleus. • Gamma rays almost always accompany alpha and beta radiation. • X rays are a form of high-energy electr ...
Radioactivity
... • γ rays do not directly ionise other atoms, although they may cause atoms to emit other particles which will then cause ionisation. – We don't find pure gamma sources - γ rays are emitted alongside alpha or beta particles. – Strictly speaking, gamma emission isn't 'radioactive decay' because it doe ...
... • γ rays do not directly ionise other atoms, although they may cause atoms to emit other particles which will then cause ionisation. – We don't find pure gamma sources - γ rays are emitted alongside alpha or beta particles. – Strictly speaking, gamma emission isn't 'radioactive decay' because it doe ...
Document
... • Gamma radiation γ : electromagnetic (EM) E that is released. • Gamma rays r (EM) waves. • no mass/charge. – Most Penetrating, can be stopped by 1m thick concrete or a several cm thick sheet of lead. ...
... • Gamma radiation γ : electromagnetic (EM) E that is released. • Gamma rays r (EM) waves. • no mass/charge. – Most Penetrating, can be stopped by 1m thick concrete or a several cm thick sheet of lead. ...
Chemistry Test: Transmutation Multiple Choice 1. Identify the new
... of 3.0 hours? a. 14.15g b. 8.0g c. 0.324g d. 2.37g Which of the following travels fastest? a. alpha particles c. gamma rays b. beta particles d. All travel at the same speed. How does the nucleus of an atom change after a gamma irradiation? a. The atomic mass reduces by four and the atomic number re ...
... of 3.0 hours? a. 14.15g b. 8.0g c. 0.324g d. 2.37g Which of the following travels fastest? a. alpha particles c. gamma rays b. beta particles d. All travel at the same speed. How does the nucleus of an atom change after a gamma irradiation? a. The atomic mass reduces by four and the atomic number re ...
Nuclear Stability
... protons is, making the nucleus unstable p atoms with atomic numbers above 82 have no stable isotopes q neutrons help to stabilize the nucleus p hydrogen is the only element that does not have neutrons p as the number of protons increases, the number of neutrons needed to keep the nucleus stable incr ...
... protons is, making the nucleus unstable p atoms with atomic numbers above 82 have no stable isotopes q neutrons help to stabilize the nucleus p hydrogen is the only element that does not have neutrons p as the number of protons increases, the number of neutrons needed to keep the nucleus stable incr ...
Nuclear - chemmybear.com
... Explain each of the following in terms of nuclear models. (a) The mass of an atom of 4He is less than the sum of the masses of 2 protons, 2 neutrons, and 2 electrons. (b) Alpha radiation penetrates a much shorter distance into a piece of material than does beta radiation of the same energy. (c) Prod ...
... Explain each of the following in terms of nuclear models. (a) The mass of an atom of 4He is less than the sum of the masses of 2 protons, 2 neutrons, and 2 electrons. (b) Alpha radiation penetrates a much shorter distance into a piece of material than does beta radiation of the same energy. (c) Prod ...
Download: Worksheet - New York Science Teacher
... A. Nuclear reactions – the energy released during nuclear reactions is much greater than the energy released during chemical reactions 1. Radioactive decay a.) The stability of an isotope is based on the ratio of neutrons to protons in its nucleus or the binding energy per nucleon. b.) Nuclei that a ...
... A. Nuclear reactions – the energy released during nuclear reactions is much greater than the energy released during chemical reactions 1. Radioactive decay a.) The stability of an isotope is based on the ratio of neutrons to protons in its nucleus or the binding energy per nucleon. b.) Nuclei that a ...
Document
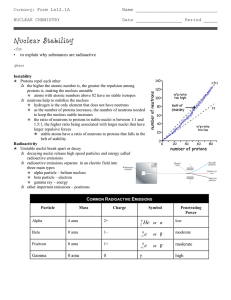
... – Alpha particles are represented by the symbols: 2 protons and 2 neutrons make a mass number of 4 it has a charge of 2+ because of the protons Alpha particles are ____________ and penetrate materials much less than the other forms of radiation. A sheet of paper will stop an alpha particle. Th ...
... – Alpha particles are represented by the symbols: 2 protons and 2 neutrons make a mass number of 4 it has a charge of 2+ because of the protons Alpha particles are ____________ and penetrate materials much less than the other forms of radiation. A sheet of paper will stop an alpha particle. Th ...
SIMPLE NUCLEAR REACTIONS
... A neutron is fired at a large nucleus (usually uranium-235). It is absorbed briefly which makes the unstable isotope of uranium-236. This then splits into two or more smaller nuclei releasing neutrons and energy in the process. The products are radioactive. Ex. uranium-235 + neutron [uranium-236] ...
... A neutron is fired at a large nucleus (usually uranium-235). It is absorbed briefly which makes the unstable isotope of uranium-236. This then splits into two or more smaller nuclei releasing neutrons and energy in the process. The products are radioactive. Ex. uranium-235 + neutron [uranium-236] ...
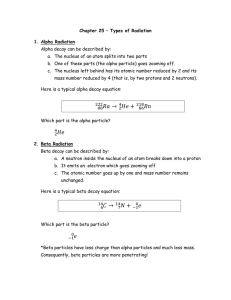
Chapter 25 – Types of Radiation 1. Alpha Radiation Alpha decay
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
nuclear force
... • A positron is emitted from the nucleus as a proton is converted into a neutron. • The atomic number decreases by one but the mass number stays the same. ...
... • A positron is emitted from the nucleus as a proton is converted into a neutron. • The atomic number decreases by one but the mass number stays the same. ...
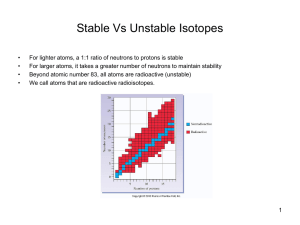
Stable Vs Unstable Isotopes
... For lighter atoms, a 1:1 ratio of neutrons to protons is stable For larger atoms, it takes a greater number of neutrons to maintain stability Beyond atomic number 83, all atoms are radioactive (unstable) We call atoms that are radioactive radioisotopes. ...
... For lighter atoms, a 1:1 ratio of neutrons to protons is stable For larger atoms, it takes a greater number of neutrons to maintain stability Beyond atomic number 83, all atoms are radioactive (unstable) We call atoms that are radioactive radioisotopes. ...
Nuclear Chemistry
... • Example: Beta decay process is the decay of iodine- 131 into xenon131 by beta-particle emission • The mass number of the product nucleus is the same as that of the original nucleus ( they are both 131), but its atomic number has increased by 1 (54 instead of 53). This changed in atomic number, and ...
... • Example: Beta decay process is the decay of iodine- 131 into xenon131 by beta-particle emission • The mass number of the product nucleus is the same as that of the original nucleus ( they are both 131), but its atomic number has increased by 1 (54 instead of 53). This changed in atomic number, and ...
Types of Radiation
... Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. (1a) Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc ...
... Students know how to relate the position of an element in the periodic table to its atomic number and atomic mass. (1a) Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc ...
Chapter 9 Natural Radioactivity
... • Slow moving, and stopped by small barriers • Symbolized in the following ways: ...
... • Slow moving, and stopped by small barriers • Symbolized in the following ways: ...
Santee Education Complex Chemistry Mini Assessment 11
... d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 15) An electron emitted from the nucleus during some kinds of radioactive decay is ...
... d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 15) An electron emitted from the nucleus during some kinds of radioactive decay is ...
PreAP Chemistry Radioactivity WS Name Period ____ Match the
... 18. Explain why the gamma rays do not bend. ...
... 18. Explain why the gamma rays do not bend. ...
Word - The Chemistry Book
... emitted from a nucleus as it changes from an excited state to a ground energy state 2. Gamma rays are produced when nuclear particles undergo transitions in energy levels; beta and gamma rays are usually emitted together 3. Gamma emission usually follows other types of decay that leave the nucleus i ...
... emitted from a nucleus as it changes from an excited state to a ground energy state 2. Gamma rays are produced when nuclear particles undergo transitions in energy levels; beta and gamma rays are usually emitted together 3. Gamma emission usually follows other types of decay that leave the nucleus i ...
Gamma ray
Gamma radiation, also known as gamma rays, and denoted by the Greek letter γ, refers to electromagnetic radiation of an extremely high frequency and therefore consists of high-energy photons. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay of atomic nuclei as they transition from a high energy state to a lower state known as gamma decay, but may also be produced by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named ""gamma rays"" by Ernest Rutherford in 1903.Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes, and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes. Additionally, gamma rays are produced by a number of astronomical processes in which very high-energy electrons are produced, that in turn cause secondary gamma rays via bremsstrahlung, inverse Compton scattering, and synchrotron radiation. However, a large fraction of such astronomical gamma rays are screened by Earth's atmosphere and can only be detected by spacecraft.Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (10−12 meter), which is less than the diameter of an atom. However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes. Electromagnetic radiation from radioactive decay of atomic nuclei is referred to as ""gamma rays"" no matter its energy, so that there is no lower limit to gamma energy derived from radioactive decay. This radiation commonly has energy of a few hundred keV, and almost always less than 10 MeV. In astronomy, gamma rays are defined by their energy, and no production process needs to be specified. The energies of gamma rays from astronomical sources range to over 10 TeV, an energy far too large to result from radioactive decay. A notable example is extremely powerful bursts of high-energy radiation referred to as long duration gamma-ray bursts, of energies higher than can be produced by radioactive decay. These bursts of gamma rays, thought to be due to the collapse of stars called hypernovae, are the most powerful events so far discovered in the cosmos.