* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download IONIZING RADIATION AND RADIONUCLIDS AS THE SOURSES …
John Gofman wikipedia , lookup
Radioactive decay wikipedia , lookup
Valley of stability wikipedia , lookup
Fallout shelter wikipedia , lookup
Radiation therapy wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Atomic nucleus wikipedia , lookup
Technetium-99m wikipedia , lookup
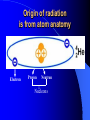
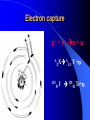
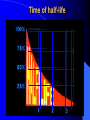
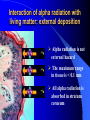
ORIGIN OF IONIZING RADIATION AND RADIONUCLIDS Historical background Humans have evolved in an environment of ionizing radiation Discovery of X rays (1895) Wilhelm Conrad Roentgen Discovery of uranium’s natural radioactivity (1896) Antoine Henri Becquerel Discovery of polonium and radium (1898) Marie Curie Discovery of harmful effects of ionizing radiation • First report about local radiation injuries (1896) and radiation-induced skin cancer (1902) • First report about radiation-induced sterility (1903) and radiation-induced leukemia (1911) • 1920s: bone cancer radium dial painters among • 1930s: liver cancer and leukemia due to Throtrast administration • 1940s: excess leukemia among first radiologists Discovery of A-bomb’s effect in Japan (1945) Hiroshima, 6.08.1945 Nagasaki, 9.08.1945 Discovery of radiation accidents consequences Coiania, Brazil (1987) Chernobyl, USSR (1986) Terrorist can use of radioactive material After September 11th, growing apprehension that by shrouding a core of conventional explosives around a radioactive source…. …..contamination could be spread over a wide area… + = …and terror created!! What is radiation? Origin of ionizing radiation Ionizing radiation Origin of radiation is from atom anatomy Electron Proton Neutron Nucleons Atomic symbols A Z MASS NUMBER (the number of protons and neutrons) XN SYMBOL OF ELEMENT The number of neutrons ATOMIC NUMBER (the number of protons) Example: 131 I 53 78 131I or I-131 Isotopes Why are some nuclides radioactive? The stable isotopes of elements have very definite ratios of neutrons to protons in their nuclei. As the atomic mass number increases, the ratio of neutrons to protons increases according to a definite pattern. If isotopes vary from this pattern, they are relatively unstable. The most stable state of a nucleus is called the ‘ground’ state. In an unstable nucleus the nucleons are in an ‘exited’ state and must release energy to reach the ground state. In the transformation of an unstable nucleus to a more stable nucleus, energy is emitted in the form of particles such as alpha and beta particles, and in some cases photons (gamma rays). This is the process of radioactive decay. Alpha (α++) decay A X Z e.g. 23892U 4 He Y + Z-2 2 A-4 234 90Th + 4 He 2 Beta (-) decay p + e- + υ n A A - + X Y +e Z Z+1 e.g. 131 I 53 13154 Xe+e-+ Positron (+) decay p A A ++ X Y+e Z Z-1 n+ e.g. + e + υ 18 F 18 O+e++ 9 8 Electron capture p+ + e- n + A A X Z Z-1 Y + 125 Te+ I 53 52 125 Gamma () emission Nuclear energy levels: gamma radiation SIMPLIFIED NUCLEAR MODEL Gamma ray Activity Radioactivity is the number of decaying nuclei per unit of time The System International (SI) unit of radioactivity is the Becquerel (Bq) 1 Bq = one disintegration per second Non-SI unit of radioactivity is the Curie (Ci) 1 Ci = 3,7 x 1010 radioactive disintegration per second 1 Bq = 2.7 x 10-11 Ci 1 Ci = 3.7 x 1010 Bq Time of half-life Electric generators of ionizing radiation Ionizing radiation can also be obtained by subjecting matter to a sufficient amount of energy. This is the principle of X-ray generators and particle accelerators X-rays can have two sources: electron rearrangement: their energy is then specific to the element considered but not to the isotope the phenomenon of incident electron retardation (Bremsstrahlung effect): their energy is non specific and varies between zero and the maximum energy as a direct function of the initial energy of the electron. Comparison of the risks from radioactive sources and electric generators The emission of ionizing radiation by a radioactive source behaves a law of decay governing the time in which work can be done Electric generators obey an “On/Off” effect which is tied to the presence or absence of the electricity supply Forms of ionizing radiation and its abilities Excitation of atom or molecules by ionizing radiation Ionization of atom or molecules by ionizing radiation Forms of ionizing radiation Directly ionizing Particulate radiation consisting of atomic or subatomic particles (electrons, protons, etc.) which carry energy in the form of kinetic energy of mass in motion Indirectly ionizing Electromagnetic radiation in which energy is carried by oscillating electrical and magnetic fields traveling through space at speed of light Specific ionization and linear energy transfer (LET) Penetrating power of radiation Alpha particle interaction Interaction of alpha radiation with living matter: external deposition Alpha radiation is not external hazard The maximum range in tissue is < 0.1 mm All alpha radiation is absorbed in stratum corneum Interaction of alpha radiation with living matter: internal deposition Prime danger is inhalation and ingestion of alpha emitter Beta interaction with matter Interaction of beta radiation with living matter Cell nucleus Cell diameter 100 cell diameter alpha 1.7 MeV beta 0.15 MeV beta beta 5.3 MeV alpha Auger I 0.001 I 0.01 I 0.1 mm I 1 I 10 ı 100 Interaction of beta radiation with living matter: external and internal deposition Beta radiation damages epithelial basal stratum. High energy ß-radiation may affect vascular layer of derma, with lesion like thermal burn Danger is inhalation and ingestion of beta emitter Danger is external ßirradiation whole body Neutron interaction Interaction of neutron radiation with living matter Neutron radiation is only external hazard: high danger of external irradiation whole body Interaction of gamma radiation with matter In terms of ionization, gamma radiation interacts with matter in three main ways: 1. Photoelectric effect 2. Compton scattering 3. Pair production Gamma interaction by photoelectric effect Gamma interaction by Compton scattering Pair production Difference between X-rays and gamma rays Interaction of gamma radiation with living matter: external and internal deposition Gamma radiation is very external hazard: - high danger of external irradiation whole body; - danger of external irradiation of skin Danger is inhalation and ingestion of gamma emitter Risk due to radiation exposure Summary of lecture • Ionizing radiation is radiation with enough energy so that during an interaction with an atom, it can remove tightly bound electrons from their orbits, causing the atom to become excitated or ionized. • Ionizing radiation occurs in two forms – particles or waves. • Alpha particles is not external hazard and can bee shielded against by clothing. Internal deposition of alpha particles is of importance on a long-term basis in terms of causing radiation injury. • Beta irradiation causes damage to the epithelial basal stratum. The lesion is similar to a superficial thermal burn. Beta particles shielding requires solid materials, like a wall. • Gamma and neutron radiation are the most biologically active, and required lead equivalent shielding for protection. • Fission products are the major radiation hazard, because a large number emit penetrating gamma radiation. This can result in whole body injuries, even at great distance. Lecture is ended THANKS FOR ATTENTION In lecture materials of the International Atomic Energy Agency (IAEA), kindly given by doctor Elena Buglova, were used