transmutation of nuclides
... emitting rays, the mass number, A, or the atomic number, Z, or both may change. When Z changes, the parent nuclide is converted to a different element. In a photon decay, the energy and other properties of the nuclide change, but neither A nor Z changes. In the transmutation, energy (including mas ...
... emitting rays, the mass number, A, or the atomic number, Z, or both may change. When Z changes, the parent nuclide is converted to a different element. In a photon decay, the energy and other properties of the nuclide change, but neither A nor Z changes. In the transmutation, energy (including mas ...
Radioactive Decays – transmutations of nuclides
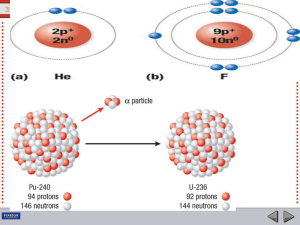
... In radioactive decay processes, some of the things are conserved, meaning they do not change. The number of nucleons before and after the decay is the same (conserved). So are electric charges and energy (including mass). The relationship between nuclides is best seen in a chart based on the number ...
... In radioactive decay processes, some of the things are conserved, meaning they do not change. The number of nucleons before and after the decay is the same (conserved). So are electric charges and energy (including mass). The relationship between nuclides is best seen in a chart based on the number ...
39 The Atomic Nucleus and Radioactivity
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. Most beta particles lose their energy during the course of a large number of glancing collisions with atomic electrons. Beta particles ...
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. Most beta particles lose their energy during the course of a large number of glancing collisions with atomic electrons. Beta particles ...
Nuclear Glossary as PDF-file
... particle, α-particle) Alpha particle Positively charged particle emitted by various radioactive materials during decay. It consists of two neutrons and two protons, and is thus identical to the nucleus of a helium atom. The rest mass of the alpha particle amounts to 6.64424·10-27 kg, or 3.7273·109 e ...
... particle, α-particle) Alpha particle Positively charged particle emitted by various radioactive materials during decay. It consists of two neutrons and two protons, and is thus identical to the nucleus of a helium atom. The rest mass of the alpha particle amounts to 6.64424·10-27 kg, or 3.7273·109 e ...
U.S. Exposures Limits: A History of Their Creation
... ○ Calcium levels are critically important for the viability of human cells. Efflux is defined as “the flowing out of a particular substance or particle.” ○ The exposure level is on the order of a SAR=0.005 Wkg8fold lower that the “safety” factor (AKA limit). Why was the “safety” factor not ...
... ○ Calcium levels are critically important for the viability of human cells. Efflux is defined as “the flowing out of a particular substance or particle.” ○ The exposure level is on the order of a SAR=0.005 Wkg8fold lower that the “safety” factor (AKA limit). Why was the “safety” factor not ...
Slide 1
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. Most beta particles lose their energy during the course of a large number of glancing collisions with atomic electrons. Beta particles ...
... A beta particle normally moves at a faster speed than an alpha particle and carries only a single negative charge. It is able to travel much farther through the air. Most beta particles lose their energy during the course of a large number of glancing collisions with atomic electrons. Beta particles ...
Radionuclides production cont.
... relationship between a long-lived parent radionuclide and its short-lived daughter radionuclide. ● In a generator, basically a long-lived parent nuclide is allowed to decay to its short-lived daughter nuclide and the latter is then chemically separated. ...
... relationship between a long-lived parent radionuclide and its short-lived daughter radionuclide. ● In a generator, basically a long-lived parent nuclide is allowed to decay to its short-lived daughter nuclide and the latter is then chemically separated. ...
Critical Thinking Questions 2
... (b) β decay: a neutron in the nucleus is converted into a proton and an electron. The electron is ejected from the nucleus. (c) Positron or β+ emission: a proton in the nucleus is converted into a neutron and a positron. The positron is ejected from the nucleus. (d) Electron capture: the nucleus cap ...
... (b) β decay: a neutron in the nucleus is converted into a proton and an electron. The electron is ejected from the nucleus. (c) Positron or β+ emission: a proton in the nucleus is converted into a neutron and a positron. The positron is ejected from the nucleus. (d) Electron capture: the nucleus cap ...
Chapter 1. Fundamentals of Atomic and Nuclear Physics
... When interactions with matter are considered, electromagnetic radiation is generally treated as series of individual particles, known as photons. The energy E of each photon is given by: ...
... When interactions with matter are considered, electromagnetic radiation is generally treated as series of individual particles, known as photons. The energy E of each photon is given by: ...
irm_ch11
... 11.25 Over 2000 bombardment-produced radionuclides that do not occur naturally are now known. 11.26 7 times greater 11.27 Uranium, element 92, has the highest atomic number of any naturally occurring element. ...
... 11.25 Over 2000 bombardment-produced radionuclides that do not occur naturally are now known. 11.26 7 times greater 11.27 Uranium, element 92, has the highest atomic number of any naturally occurring element. ...
Ionizing radiation
Ionizing (or ionising in British English) radiation is radiation that carries enough energy to free electrons from atoms or molecules, thereby ionizing them. Ionizing radiation is made up of energetic subatomic particles, ions or atoms moving at relativistic speeds, and electromagnetic waves on the high-energy end of the electromagnetic spectrum.Gamma rays, X-rays, and the higher ultraviolet part of the electromagnetic spectrum are ionizing, whereas the lower ultraviolet part of the electromagnetic spectrum, visible light (including nearly all types of laser light), infrared, microwaves, and radio waves are considered non-ionizing radiation. The boundary between ionizing and non-ionizing electromagnetic radiation that occurs in the ultraviolet is not sharply defined, since different molecules and atoms ionize at different energies. Conventional definition places the boundary at a photon energy between 10 eV and 33 eV in the ultraviolet (see definition boundary section below).Typical ionizing subatomic particles from radioactivity include alpha particles, beta particles and neutrons. Almost all products of radioactive decay are ionizing because the energy of radioactive decay is typically far higher than that required to ionize. Other subatomic ionizing particles which occur naturally are muons, mesons, positrons, neutrons and other particles that constitute the secondary cosmic rays that are produced after primary cosmic rays interact with Earth's atmosphere. Cosmic rays may also produce radioisotopes on Earth (for example, carbon-14), which in turn decay and produce ionizing radiation.Cosmic rays and the decay of radioactive isotopes are the primary sources of natural ionizing radiation on Earth referred to as background radiation.In space, natural thermal radiation emissions from matter at extremely high temperatures (e.g. plasma discharge or the corona of the Sun) may be ionizing. Ionizing radiation may be produced naturally by the acceleration of charged particles by natural electromagnetic fields (e.g. lightning), although this is rare on Earth. Natural supernova explosions in space produce a great deal of ionizing radiation near the explosion, which can be seen by its effects in the glowing nebulae associated with them.Ionizing radiation can also be generated artificially using X-ray tubes, particle accelerators, and any of the various methods that produce radioisotopes artificially.Ionizing radiation is invisible and not directly detectable by human senses, so radiation detection instruments such as Geiger counters are required. However, ionizing radiation may lead to secondary emission of visible light upon interaction with matter, such as in Cherenkov radiation and radioluminescence.Ionizing radiation is applied constructively in a wide variety of fields such as medicine, research, manufacturing, construction, and many other areas, but presents a health hazard if proper measures against undesired exposure aren't followed. Exposure to ionizing radiation causes damage to living tissue, and can result in mutation, radiation sickness, cancer, and death.