Chapter 37
... – Beta rays (b) bend in the opposite direction indicating they have a negative charge. ...
... – Beta rays (b) bend in the opposite direction indicating they have a negative charge. ...
Nuclear Chemistry
... What happens when a positron collides with an electron? Annihilation!! This can be shown by the following reaction: Example : 0-1e + 01e ----> 2 Gamma Rays As the name implies, these are not particles but high energy photons and can be found on the electromagnetic spectrum. They are very similar to ...
... What happens when a positron collides with an electron? Annihilation!! This can be shown by the following reaction: Example : 0-1e + 01e ----> 2 Gamma Rays As the name implies, these are not particles but high energy photons and can be found on the electromagnetic spectrum. They are very similar to ...
Mubarak-Report - KFUPM Faculty List
... about 11 MeV, a process known as prompt gamma radiation. At this stage the nucleus can either become a stable nucleus again or a radioactive one. The radioactive nucleus so formed now decays and furthers producing beta particles and a cascade of gamma rays known as delayed gamma radiation. Neutron ...
... about 11 MeV, a process known as prompt gamma radiation. At this stage the nucleus can either become a stable nucleus again or a radioactive one. The radioactive nucleus so formed now decays and furthers producing beta particles and a cascade of gamma rays known as delayed gamma radiation. Neutron ...
Nuclear Decay - Physics Rocks!
... – Essentially the same result as positron emission – An electron from the lowest energy level is “captured” by the nucleus, turning a proton into a neutron ...
... – Essentially the same result as positron emission – An electron from the lowest energy level is “captured” by the nucleus, turning a proton into a neutron ...
Nuclear Decay - Issaquah Connect
... – Essentially the same result as positron emission – An electron from the lowest energy level is “captured” by the nucleus, turning a proton into a neutron ...
... – Essentially the same result as positron emission – An electron from the lowest energy level is “captured” by the nucleus, turning a proton into a neutron ...
01 physical, technological and organizational bases of radiation
... • Neutron radiation consists of neutrons that are ejected from the nuclei of atoms. A neutron has no electrical charge. Due to their charge, neutrons do not interact directly with electrons in matter. A direct interaction occurs as the result of a "collision" between a neutron and the nucleus of an ...
... • Neutron radiation consists of neutrons that are ejected from the nuclei of atoms. A neutron has no electrical charge. Due to their charge, neutrons do not interact directly with electrons in matter. A direct interaction occurs as the result of a "collision" between a neutron and the nucleus of an ...
Nuclear Reactions - Manasquan Public Schools
... Gamma Radiation Gamma rays are extremely penetrating making them dangerous. ...
... Gamma Radiation Gamma rays are extremely penetrating making them dangerous. ...
31.1 Nuclear Structure
... Example 3 The Binding Energy of the Helium Nucleus Revisited The atomic mass of helium is 4.0026u and the atomic mass of 1hydrogen isotope is 1.0078u. Using atomic mass units, instead of kilograms, obtain the binding energy of the helium nucleus. ...
... Example 3 The Binding Energy of the Helium Nucleus Revisited The atomic mass of helium is 4.0026u and the atomic mass of 1hydrogen isotope is 1.0078u. Using atomic mass units, instead of kilograms, obtain the binding energy of the helium nucleus. ...
Nuclear Chemistry - Duplin County Schools
... The answer is A. The largest source of background radiation is from the decay of radon gas, produced by the alpha decay of uranium-238. ...
... The answer is A. The largest source of background radiation is from the decay of radon gas, produced by the alpha decay of uranium-238. ...
Introduction to Radiation Physics, Quantities and Units
... • Charged particles are emitted from the atomic nucleus at high energy in some nuclear transformations. These include alpha and beta particles. • Uncharged particles (neutrons) are produced by fission or other nuclear reactions. • Both types of particles produce ionization. ...
... • Charged particles are emitted from the atomic nucleus at high energy in some nuclear transformations. These include alpha and beta particles. • Uncharged particles (neutrons) are produced by fission or other nuclear reactions. • Both types of particles produce ionization. ...
Radioactive Elements (pages 139–146)
... • A nuclear reaction is a reaction that involves the nucleus of an atom. A nuclear reaction can change an element into a different element. • Some isotopes of elements break apart naturally. Radioactive decay is a process in which the nuclei of unstable isotopes give off atomic particles and energy. ...
... • A nuclear reaction is a reaction that involves the nucleus of an atom. A nuclear reaction can change an element into a different element. • Some isotopes of elements break apart naturally. Radioactive decay is a process in which the nuclei of unstable isotopes give off atomic particles and energy. ...
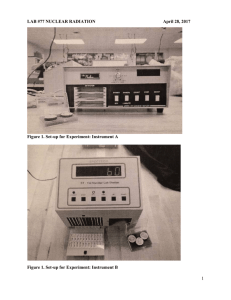
Lab 77 Nuclear Radiation Detection
... is inversely related to the square of the distance from the radiation source. This is known as the inverse square law. All forms of radiation follow this law. Therefore, unless you know where a radiation source is located, you need a sensitive device to detect it. A common method of detecting alpha ...
... is inversely related to the square of the distance from the radiation source. This is known as the inverse square law. All forms of radiation follow this law. Therefore, unless you know where a radiation source is located, you need a sensitive device to detect it. A common method of detecting alpha ...
Absorption and Biological Effects of Ionising Radiation
... penetrating than alpha particles, and can travel several metres in air. Beta particles are less ionising than alpha particles, but due to their greater penetrating ability, they can damage some external tissues directly. ...
... penetrating than alpha particles, and can travel several metres in air. Beta particles are less ionising than alpha particles, but due to their greater penetrating ability, they can damage some external tissues directly. ...
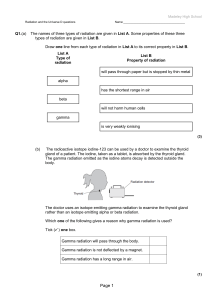
Page 1 - Madeley High School
... The doctor uses an isotope emitting gamma radiation to examine the thyroid gland rather than an isotope emitting alpha or beta radiation. Which one of the following gives a reason why gamma radiation is used? Tick ( ) one box. Gamma radiation will pass through the body. Gamma radiation is not deflec ...
... The doctor uses an isotope emitting gamma radiation to examine the thyroid gland rather than an isotope emitting alpha or beta radiation. Which one of the following gives a reason why gamma radiation is used? Tick ( ) one box. Gamma radiation will pass through the body. Gamma radiation is not deflec ...
II. Basic Physics of Ionizing Radiation
... • Radiation can be thought of as the transmission of energy through space. • Two major forms of radiation: – Electromagnetic (EM) radiation – Particulate radiation ...
... • Radiation can be thought of as the transmission of energy through space. • Two major forms of radiation: – Electromagnetic (EM) radiation – Particulate radiation ...
Introduction to Nuclear Radiation Introduction.
... Beta particles (electrons or positrons), in contrast to alpha particles, are emitted in a given decay with energies from zero up to a definite maximum (which is usually less than alpha particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interact ...
... Beta particles (electrons or positrons), in contrast to alpha particles, are emitted in a given decay with energies from zero up to a definite maximum (which is usually less than alpha particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interact ...
ch10_sec1_rc
... • Gamma rays are high-energy electromagnetic radiation. • gamma ray: a high-energy photon emitted by a nucleus during fission and radioactive decay • When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely st ...
... • Gamma rays are high-energy electromagnetic radiation. • gamma ray: a high-energy photon emitted by a nucleus during fission and radioactive decay • When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely st ...
Nuclear Chemistry
... All nuclear decay is accompanied by a release of energy. Alpha and beta particles have ...
... All nuclear decay is accompanied by a release of energy. Alpha and beta particles have ...
Radioactivity presentation script
... that they can ionize matter. We can use this to build a detector for ionizing radiation. There are many types of detectors, but the type we used on Friday is one of the simplest and most effective - the Geiger-Müller tube. The diagram on the screen shows a schematic of a GM tube. The tube consists o ...
... that they can ionize matter. We can use this to build a detector for ionizing radiation. There are many types of detectors, but the type we used on Friday is one of the simplest and most effective - the Geiger-Müller tube. The diagram on the screen shows a schematic of a GM tube. The tube consists o ...
Introduction to Nuclear Radiation
... Beta particles (electrons or positrons), in contrast to alpha particles, are emitted in a given decay with energies from zero up to a definite maximum (which is usually less than alpha particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interact ...
... Beta particles (electrons or positrons), in contrast to alpha particles, are emitted in a given decay with energies from zero up to a definite maximum (which is usually less than alpha particle energies). Except for very high energy beta rays (greater than several MeV) the main slowing-down interact ...
Radioactivity Revision Questions Decay – Nucleus
... 1. What is Radioactivity? Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting partic ...
... 1. What is Radioactivity? Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting partic ...
Radioactive Decay
... Positron emission : a particle that has the same mass as an electron, but has a positive charge and is emitted during some types of radioactive decay. ...
... Positron emission : a particle that has the same mass as an electron, but has a positive charge and is emitted during some types of radioactive decay. ...
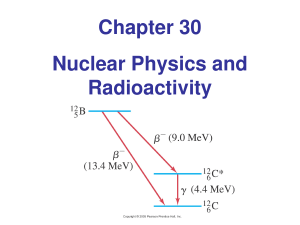
Chapter 30 Nuclear Physics and Radioactivity
... Neutrinos are notoriously difficult to detect, as they interact only weakly, and direct evidence for their existence was not available until more than 20 yrs had passed after they were ‘predicted’. The symbol for the neutrino is the Greek letter nu, ν We can write the beta decay of carbon14 as ...
... Neutrinos are notoriously difficult to detect, as they interact only weakly, and direct evidence for their existence was not available until more than 20 yrs had passed after they were ‘predicted’. The symbol for the neutrino is the Greek letter nu, ν We can write the beta decay of carbon14 as ...
Gamma ray
Gamma radiation, also known as gamma rays, and denoted by the Greek letter γ, refers to electromagnetic radiation of an extremely high frequency and therefore consists of high-energy photons. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay of atomic nuclei as they transition from a high energy state to a lower state known as gamma decay, but may also be produced by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named ""gamma rays"" by Ernest Rutherford in 1903.Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes, and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes. Additionally, gamma rays are produced by a number of astronomical processes in which very high-energy electrons are produced, that in turn cause secondary gamma rays via bremsstrahlung, inverse Compton scattering, and synchrotron radiation. However, a large fraction of such astronomical gamma rays are screened by Earth's atmosphere and can only be detected by spacecraft.Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (10−12 meter), which is less than the diameter of an atom. However, this is not a hard and fast definition, but rather only a rule-of-thumb description for natural processes. Electromagnetic radiation from radioactive decay of atomic nuclei is referred to as ""gamma rays"" no matter its energy, so that there is no lower limit to gamma energy derived from radioactive decay. This radiation commonly has energy of a few hundred keV, and almost always less than 10 MeV. In astronomy, gamma rays are defined by their energy, and no production process needs to be specified. The energies of gamma rays from astronomical sources range to over 10 TeV, an energy far too large to result from radioactive decay. A notable example is extremely powerful bursts of high-energy radiation referred to as long duration gamma-ray bursts, of energies higher than can be produced by radioactive decay. These bursts of gamma rays, thought to be due to the collapse of stars called hypernovae, are the most powerful events so far discovered in the cosmos.