* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Drugs - BIDD - National University of Singapore
NMDA receptor wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
CCR5 receptor antagonist wikipedia , lookup
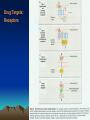
Discovery and development of beta-blockers wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
Prescription costs wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Pharmacognosy wikipedia , lookup
Toxicodynamics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Drug discovery wikipedia , lookup
Psychopharmacology wikipedia , lookup
Drug design wikipedia , lookup
Drug interaction wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
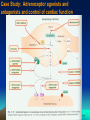
Nicotinic agonist wikipedia , lookup
CZ5225: Modeling and Simulation in Biology Lecture 2: Drugs Prof. Chen Yu Zong Tel: 6874-6877 Email: [email protected] http://xin.cz3.nus.edu.sg Room 07-24, level 7, SOC1, National University of Singapore Definitions Xenobiotic: A chemical that is not endogenous to an organism. Endogenous: Made within. Drug: A chemical taken that is intended to modulate the current physiological status quo. Ligand: A chemical that binds to another molecule, such as a receptor protein. Bioavailability: The amount or proportion of drug that becomes available to the body following its administration. Pharmacokinetics: What the body does to a drug. Pharmacodynamics: What a drug does to the body . 2 Drug action A drug is a compound that can modify the response of a tissue to its environment. A drug will exert its activity through interactions at one or more molecular targets. • The macromolecular species that control the functions of cells. • May be surface-bound proteins like receptors and ion channels or • Species internal to cells, such as enzymes or nucleic acids. 3 Drug-Receptor Lock and Key Model 4 Drug Targets: Receptors Receptors are the sites at which biomolecules such as hormones, neurotransmitters and the molecules responsible for taste and odour are recognised. A drug that binds to a receptor can either: • Trigger the same events as the native ligand - an agonist. Or • Stop the binding of the native agent without eliciting a response - an antagonist. There are four ‘superfamilies’ of receptors. 5 Drug Targets: 6 Drug Targets: Receptors 7 Drug Targets: Receptors 8 Drug Targets: Receptors 9 Drug Targets: Enzymes They are proteins that catalyse the reactions required for cellular function. Generally specific for a particular substrate, or closely related family of substrates. Molecules that restrict the action of the enzyme on its substrate are called inhibitors. Inhibitors may be irreversible or reversible. Reversible inhibitors may be: • Competitive. • Non-competitive. Enzyme inhibitors might be seen to allow very ‘fine control’ of cellular processes. 10 Drug targets: Nucleic acids Potentially the most exciting and valuable of the available drug targets. BUT designing compounds that can distinguish target nucleic acid sequences is not yet achievable. There are compounds with planar aromatic regions that bind inbetween the base pairs of DNA or to the DNA grooves. These generally inhibit the processes of DNA manipulation required for protein synthesis and cell division. • Suitable as drugs for applications where cell death is the goal of therapy - such as in the case of the treatment of cancer. • Name another use where cell death is desirable. 11 Mechanisms and Specificity of Drug Binding The majority of binding and recognition occurs through noncovalent interactions. These govern: • The folding of proteins and DNA. • The association of membranes. • Molecular recognition (e.g. interaction between an enzyme and its substrate or the binding of an antibody). They are generally weak and operate only over short distances. As a result large numbers of these interactions are necessary for stability, requiring a high degree of complementarity between binding groups and molecules. 12 Drug Binding Site HIV-1 protease 13 Mechanism of Drug Binding and Actions Drug and protein: Lock and key mechanism, blocking=>stopping of protein function 14 Protein Surface X-Ray Diffraction Structure Of Hiv-1 Protease Complexed With SB203238 (Drawn from: Brookhaven database file: 1hbv.pdb. K.A.Newlander, J.F.Callahan, M.L.Moore, T.A.Tomaszek, W.F.Huffman A Novel Constrained Reduced-Amide Inhibitor Of HIV-1 Protease Derived From The Sequential Incorporation Of Gamma-Turn Mimetics Into A Model Substrate J.Med.Chem. 1993, 36, 2321.) 15 Covalent bonds The ‘sharing’ of a pair of electrons between two atoms. These electrons largely occupy the space between the nuclei of the two atoms. • A very stable interaction • Requires hundreds of kilojoules to disrupt. Compounds that inhibit enzymes through formation of covalent interactions are called ‘suicide inhibitors’. Not all covalent bond formation is irreversible • Hydrolysis. • Action of repairing proteins. Consult with your Biochemistry textbook 16 Non-covalent interactions The forces involved are: • Hydrogen bonds • van der Waals forces • Ionic / electrostatic interactions • Hydrophobic interactions. Generally, such interactions are weak •vary from 4-30 kJ/mol. Details later Selectivity, toxicity and therapeutic index Drugs may bind to both their desired target and to other molecules in an organism. If interactions with other targets are negligible then a drug is said to be specific. In most cases drugs will show a non-exclusive preference for their target - selective. The interaction with both their intended target and other molecules can lead to undesirable effects (side effects). 18 Selectivity, toxicity and therapeutic index Establish the concentrations at which the drug exerts its beneficial effect and where the level of side effects becomes unacceptable. Commonly used values are ED50 and LD50. For obvious reasons LD50 tests are not carried out on human volunteers! One measure of the margin of safety is the therapeutic index. Therapeutic index = LD50 / ED50 Drugs with low therapeutic indices are only used in ‘life or death’ type situations. Exercise: it can be argued that the ratio LD1 / ED99 might be a more realistic estimate of safety. Why? 19 Agonists & antagonists Activity of a drug is the result of two independent factors: • Affinity is the ability of a drug to bind to its receptor. • Efficacy describes the ability of the bound drug to elicit a response. The ‘two state model’. Receptors can be inactive or activated. An agonist stabilises the active state preferentially. An antagonist shows no preference or it stabilises the resting state. 20 Agonists & antagonists Activity of a drug is the result of two independent factors: • Affinity is the ability of a drug to bind to its receptor. • Efficacy describes the ability of the bound drug to elicit a response. The ‘two state model’. Receptors can be inactive or activated. An agonist stabilises the active state preferentially. An antagonist shows no preference or it stabilises the resting state. 21 Agonists & antagonists The efficacy of a compound in the two state model is the degree of selectivity for stabilising the active or resting state of the receptor. The degree of selectivity can be expressed in terms of the ratio of the equilibrium binding constant, K for each receptor state. • Kactive / Kresting > 1, then the compound is an agonist. The higher the ratio, the higher will be the efficacy. • Kactive / Kresting 1, then the compound is an antagonist. The smaller the ratio, the higher will be the efficacy. 22 Agonists & antagonists There are 2 classes of agonist: • Full agonists – which elicit the maximum possible response at some concentration • Partial agonists – which never elicit the maximum possible response from the receptor. 23 Agonists & antagonists There are also 2 classes of antagonist: • Competitive antagonists – which compete for the agonist binding site, and require higher agonist concentration to elicit a given response. • Non-competitive agonists – these bind at a site other than the agonist binding site, or even to a completely different molecular target. The result is the lowering of the maximum possible response in addition to the usual antagonist effect of ‘displacing’ agonist activity to higher concentration. 24 Case Study: Adrenoceptor agonists and antagonists and control of cardiac function 25 Case Study: Adrenoceptor agonists and antagonists and control of cardiac function Adrenoceptors The receptors for adrenaline (epinephrine) and noradrenaline (norepinephrine). Also called adrenergic receptors. Widely distributed, being responsible for control of the stimulation and relaxation of muscle, including the heart. Adrenoceptors mediate the control of cardiac function by the sympathetic nervous system; the parasympathetic nervous system control is mediated by muscarinic acetylcholine receptors. 26 NH2 CO2H HO NH2 HO CO2H HO Tyrosine DOPA OH NH2 HO NH2 HO HO HO Norepinephrine OH HO Dopamine Route of the biosynthesis of epinephrine and norepinephrine H N CH3 HO Epinephrine 27 Case Study: Adrenoceptor agonists and antagonists and control of cardiac function Adrenoceptors are divided into 5, or possibly 6 types: 1, 2, 1, 2, 3, and potentially 4. They are all G-protein coupled receptors. The secondary messengers for the 1 adrenoceptors are inositol triphosphate and diacylglycerol. All other adrenoceptors have cAMP as their principal secondary messenger. Remember that cytoplasmic [Ca2+] regulates the development of tension in muscles, such as the heart. 28 Case Study: Adrenoceptor agonists and antagonists and control of cardiac function 29 Case Study: Adrenoceptor agonists and antagonists and control of cardiac function The activation of and adrenoceptors usually elicits opposing responses: • receptor activation leads to constriction of veins and arterioles. • receptor activation leads to dilation of veins and arterioles. 30 Presence and function of adrenoceptors and the heart and vascular system. Epinephrine administered rapidly intravenously has a number of simultaneous effects that contribute to a rapid rise in blood pressure on its administration. • A rise in the strength of ventricular contraction (a positive inotropic action) • The heart rate is increased (a positive chronotropic action) • Blood vessels become constricted. Noting the opposing roles of and receptors, it may be no surprise to discover that administration regimes other than rapidly intravenous injection can have quite different effects. 31 Adrenoceptors: Signalling Process 32 Adrenoceptors: Signalling Process 33 1-Adrenoceptors: These are less abundant than adrenoceptors. They couple to phospholipases C and D, to certain Ca2+ channels, and a number of ion channels allowing modification of cellular cation content, including K+ and Na+. Stimulation of 1-adrenoceptors does not lead to elevated cAMP levels within the cell, and may even reduce cAMP levels. 1-Adrenoceptor stimulation leads to formation of 1,4,5inositoltriphosphate and diacylglycerol. 34 1-Adrenoceptors: Inositoltriphosphate releases Ca2+ from intracellular stores, and this may explain the observed increase in force of contraction upon 1-adrenoceptor activation. Their activation leads to constriction of vascular smooth muscle. 35 2-Adrenoceptors: Present in only very low levels in the heart. Their activation leads to constriction of vascular smooth muscle. 1 and 2-Adrenoceptors: The ratio of 1 to 2-Adrenoceptors is about 65:35 in the atria, and around 75:25 in the ventricles. These receptors both lead to increases of [cAMP] following stimulation. This in turn activates protein kinase A, which can phosphorylate, amongst other proteins, certain Ca2+ channels, leading to an influx of Ca2+ ions, and so enhances contraction. -Adrenoceptor agonists also increase heart rate. 36 Only the 1 receptor is thought to be involved in the exerciseinduced increase in heart rate bought about by noradrenaline. Adrenaline, on the other hand, may function primarily through the 2-adrenoceptors. 2-adrenoceptor activation also leads to relaxation of vascular smooth muscle. 3, and potentially 4-Adrenoceptors: The presence of these in the heart is not fully established, and their role, if present, is even more uncertain. 37 Adrenoceptor agonists 1-Adrenoceptor agonists: These can be used to treat hypotension through vasoconstriction, leading to increased blood pressure and cardiac arrhythmias through activation of vagal reflexes. Also valuable adjuncts to local anaesthetics, as vasoconstriction can slow the systemic dispersal of the anaesthetic. Drugs in this class include phenylephrine and methoxamine. 38 Adrenoceptor agonists 2-Adrenoceptor agonists: Despite the tendency of adrenoceptor agonists to cause vasoconstriction, these can be used to treat hypertension. This unexpected activity occurs through action at the CNS, reducing signal to the heart and so lowering cardiac activity and constriction of the peripheral vasculature. Drugs in this class include methyldopa and clonidine. Clonidine can also be used in protection against migrane. 39 -Adrenoceptor agonists: • These can be used to treat hypotension, cardiac arrhythmias and cardiac failure. • They stimulate the rate and force of cardiac contraction. • Simultaneously, they lead to a drop in peripheral vascular resistance. • These combined effects can result in palpitations, sinus tachycardia and serious arrhythmias. 40 -Adrenoceptor agonists: Drugs in this class include xamoterol and dobutamine. 2-Adrenoceptor agonists lead to muscle relaxation and so find use in treatment of asthma (salbutamol) and delay in the onset of labour. (ritodrine). 41 Adrenoceptor antagonists 1-Adrenoceptor antgonists: Antagonism (or ‘blockade’) of 1-adrenoceptors inhibits the action of endogenous vasoconstrictors, resulting in vasodilation of both arteries and veins, and thus reduction of blood pressure. These drugs are, therefore, useful in the treatment of hypertension and cardiac failure. Prazosin and indoramin fall into this class of compounds. 42 Adrenoceptor antagonists 2-Adrenoceptor antagonists: Just as 2adrenoceptor agonists unexpectedly reduce vasoconstriction and lower cardiac activity, their antagonists cause a rise in blood pressure through reversal of these effects. Yohimbine is an 2-adrenoceptor antagonist. 43 -Adrenoceptor antagonists: These can be used to treat hypertension, angina, cardiac arrhythmias and ischemic heart disease. The effects of -adrenoceptor antagonists (‘-blockers’) are only evident when the heart is under stress or increased workload. Under these circumstances, they preclude or attenuate increases in the rate and force of cardiac contraction. They also cause an increase in peripheral resistance to blood flow, although this effect is reversed on prolonged administration. Drugs in this class include propanolol and metoprelol. 44