* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Transgenic Animals and Plants
Nutriepigenomics wikipedia , lookup
Genetically modified food wikipedia , lookup
Point mutation wikipedia , lookup
Genetically modified organism containment and escape wikipedia , lookup
Gene nomenclature wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
X-inactivation wikipedia , lookup
Gene expression profiling wikipedia , lookup
Genome (book) wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genetically modified crops wikipedia , lookup
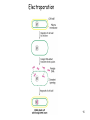
Mir-92 microRNA precursor family wikipedia , lookup
Genome editing wikipedia , lookup
Gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
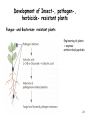
Microevolution wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Designer baby wikipedia , lookup
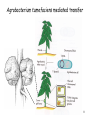
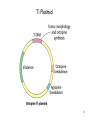
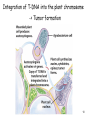
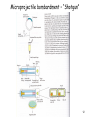
Transgenic Animals and Plants - Genetic Engineering of plant -> Transgenic plants - Genetic Engineering of animals -> Transgenic animals 1 Definition of Transgenic Transgenic -> stable introduction of a gene into another organism -> For Unicellular organisms (such as bacteria or yeast) all transformed cells are -> transgenic -> For multicellular organisms (such as animals, plants,..) difference between: - manipulation of single cells -> cell line (expression in insect cells or mammalian cells) - manipulation of a whole plant or animal -> transgenic (can have a transgenic offspring!!!) -> more difficult and expensive to create whole modified organism (transgenic) than just cell line!!! 2 Transgenic versus Cloning Transgenic -> creation of transgenic animal or plant (introduction of foreign gene into organism) -> transgenic organisms produced by introduction of foreign gene into germ line (-> transgenic offspring!!!) -> introduction of gene into somatic cells -> gene therapy Cloning -> obtaining an organism that is genetically identical to the original organism -> such as Dolly the sheep -> asexual propagation of plants (taking cuttings) 3 Transgenic Plants Why do we need transgenic plants ? • improvement of agricultural value of plant (resistance to herbicides, resistance to insect attack -> Bacillus thuringiensis toxin) • living bioreactor -> produce specific proteins • studying action of genes during development or other biological processes (knock-out plants, expression down-regulated) 4 Transgenic Plants • Advantages: - Plant cells are totipotent -> whole plant can be regenerated from a single cell (engineered cells -> engineered plants) - Plants have many offspring -> rare combinations and mutations can be found - Transposons used as vectors • Disadvantages: - Large genomes (polypoid -> presence of many genomes in one cell) - plants regenerating from single cells are not genetically homogenous (genetically instable) 5 6 Gene – transfer methods 7 Agrobacterium tumefaciens mediated transfer 8 Ti Plasmid 9 Integration of T-DNA into the plant chromosome -> Tumor formation 10 Gene transfer by cointegration Recombinant Ti plasmid by recombination 11 Microprojectile bombardment – “Shotgun” 12 Viral Vectors 13 Transfer into protoplasts Vector + polyethylene glycol Gene transfer across a protoplast membrane is promoted by some chemicals such as polyethylene glycol 14 Electroporation 15 Control elements on vector Frequently used promoter: -> 35S promoter from cauliflower mosaic virus 16 Alterations in plant RNA – downregulation of specific genes PG (polygalacturonase) -> Sensitivity of tomatoes to bruising Reduced level-> should give harder fruit during shipping Result: lower level -> did not give harder fruit (more factors responsible for process) Expression of Antisense RNA of transcript of PG -> reduces level of protein produced 17 Selection marker free transgenic plant -> Transposons 18 Applications for engineering plants • Development of Insect-, pathogen-, herbicide- resistant plants • Flower pigmentation • Modification of nutritional content • Modification of taste and appearance • Bioreactor • Vaccines (Cholera toxin-like protein in potatoes) • Plant yield (alteration of lignin content -> paper industry) 19 Development of Insect-, pathogen-, herbicide- resistant plants Toxin from Bacillus thuringiensis 20 Development of Insect-, pathogen-, herbicide- resistant plants 21 Development of Insect-, pathogen-, herbicide- resistant plants Manipulations that make a plant herbicide resistance - Inhibit the uptake of the herbicide - overproduce the herbicide-sensitive target protein (Glyphosate) - reduce ability of target protein to bind herbicide (cyclohexanediones) - plant can degrade herbicide (Bromoxynil, Glufosinate, Cyanamide,..) 22 Development of Insect-, pathogen-, herbicide- resistant plants Fungus- and Bacterium- resistant plants Engineering of plants -> express antimicrobial peptides 23 Flower pigmentation CHS -> Chalone synthetase -> enzyme in biosynthetic pathway of a purple pigment 24 Changed nutrition content - Amino acids (to increase lysine content in the future in animal food) - Lipids (possible to change degree of unsaturation, chain length) - Vitamins (Vitamin E, increase Vitamin A in rice) 25 Modification of taste and appearance Engineer potatoes -> produce more glucose and fructose at higher temperatures 26 Plants as bioreactor -Therapeutic agents - Antibodies - polymers (PHB) 27 Transgenic Animals Transgene -> Gene coding for a growth hormone 28 Transgenic Animals Why do we need transgenic animals ? • living bioreactor -> produce specific proteins in the milk (cattle, sheep, goats, pigs) • studying action of genes during development or other biological processes (knock-out animals, expression down-regulated) -> models for studying human diseases -> mice • improvement of agricultural value (fish, bird) 29 Gene-transfer methods • Microinjection • Retroviral method • Engineered Embryonic Stem Cells (ES) method • Knock – out methods (Cre-LoxP system) -> studying gene expression + development 30 The first days of an embryo Used for retroviral infection Fertilized egg Embryonic stem cells (ES) 31 Microinjection into the germ line -> transgenic animal Gene injected into the male pronuclei 32 Efficiency of the transgenesis process after DNA microinjection 33 Retroviral vectors into the germ line (8-cell embryo infected) -> transgenic animal 34 Engineered Embryonic Stem Cells (ES) into the germ line (blastocyst) -> transgenic animal Engineered ES -> can form any kind of cell in an embryo Inner cell mass (ICM) of blastocysts can form all cells of the embryo -> Pluripotent -> Embryonic stem cells 35 Gene Therapy – Viral gene transfer into somatic cells Gene transfer into somatic stem cells -> gene therapy Gene transfer via Virus Target tissues: Bone marrow, liver, brain,.... 36 Gene Therapy – Viral gene transfer into somatic cells Gene transfer into somatic stem cells -> gene therapy Used for treating -> genetic diseases, such as diabetes, cancer, color blindness… Different delivery methods 37 Gene Transfer - what happens on DNA level Integration into chromosome -> Recombinantion Recombinantion can be -> homologous – non-homologous - non-homologous event -> more frequently - homologous event -> less frequent but desired Knock-out mutants -> disrupt functional gene by integration of another gene into target gene Used for: -> study human diseases by creating model organisms -> make minus mutant 38 Homologous recombinantion 39 How do check for homologous recombinantion 40 Construction of a disruption construct 41 42 43 44 Cre-LoxP system: - Inactivation of a gene (knock-out) in a specific cell type - Activation of a transgene in specific cell type Used for: - Study biological consequences of tissue- specific gene inactivation -> establishing models for human diseases -> selective removal of kinesin II gene (expressed in retinal receptor cells) -> leads to accumulation of opsin and arrestin -> cell death -> result mimics aspects of a disease (inherited retinis pigmentosa) -> large deletions in chromosome -> deletion in chr. 22 -> DiGeorge syndrome (cardiovascular dysfunction) 45 Inactivation of gene in specific cell type (tissue) 46 Cloning of Dolly – Cloning Animals by Nuclear Transfer Technology Critical for success: Cell cycle of the somatic cells (udder cells) on plates was critical – they were kept in specific growth stage (diploid stage) Until 1997, arrival of Dolly – not possible to produce an adult animal from a nucleus from an adult animal´s differentiated cell Of the 434 fused oocytes created during the experiment -> only Dolly survived to adulthood Dolly was real clone (genotype identical) and could reproduce Dolly was euthanized 2003 -> suffering from progressive lung disease Since 1997 -> cloning of sheep, cows, mice, cats, other animals done -> many of the clones developed severe diseases as they matured. 47 Cloning of Mammals – Reproductive Cloning - Genotype identical - Phenotype is not necessarily identical -> variation due to random events and due to environment 48 Why do clones have health problems? Telomeres are found at the end of each chromosome. Shrinking of the telomeric ends of our chromosomes are a sign of aging of the cell. Each cycle of cell division the telomeres are slightly shortened until they are too short for further replication -> cell death Dolly´s telomeres (at the age of 3) have been as short as ones of the age of 6 -> clones age “faster”. 49 Why do clones have health problems? Differentiated cells have certain methylation pattern. Cloned animals have abnormal methylation pattern originating from nucleus from differentiated cells Some can be “re-set” (epigenetic reprogramming) to their undifferentiated state, some cannot -> faulty gene activation in cloned animal -> so few cloned embryos survive -> surviving clones have severe health problems 50 Transgenic Cattle, Sheep, Goat, Pigs Production of pharmaceutical proteins -> drugs Problems: Highly inefficient Only 20% of the eggs survive and only 5% of them produce product 51 Transgenic Cattle, Sheep, Goat, Pigs - Protein production: in milk, blood, urin - Animals (pigs) with modification of sugars on surface of organs -> donor for organ transplants 52 Transgenic Cattle, Sheep, Goat, Pigs 53 Transgenic birds and fish -> improvement of agricultural value Transgenic chicken: - Resistant to viral, bacterial diseases - better feeding efficiency (fast growth, better meat quality, more meat - less fat meat, less cholesterol in eggs - maybe use of eggs as bioreactors for protein production Transgenic fish: -> to support aquaculture - Increase growth rate (growth hormone) - resistance to diseases - Generation of model systems to monitor health hazard (screening chemicals if they cause mutations) 54