* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File - Mrs Jones A
Photosynthetic reaction centre wikipedia , lookup
Genetic code wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Electron transport chain wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Butyric acid wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
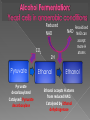
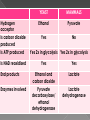
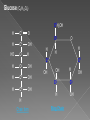
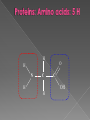
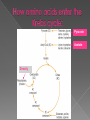
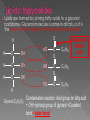
Remind yourselves using whiteboards of alcoholic and lactate fermentation You are then going to compare anaerobic respiration in yeast and mammals Reduced NAD CO2 Pyruvate Pyruvate decarboxylated Catalysed: Pyruvate decarboxylase 2H Ethanal Reoxidised NAD NAD can accept more H atoms Ethanol Ethanal accepts H atoms from reduced NAD. Catalysed by Ethanol dehydrogenase Reduced NAD Pyruvate is the H acceptor (H atoms) from reduced NAD NAD is now reoxidised and can accept more H atoms from glucose SO GLYCOLYSIS CAN CONTINUE NAD 2H Pyruvate Lactate Catalysed by Lactate dehydrogenase YEAST Hydrogen acceptor Is carbon dioxide produced Is ATP produced Is NAD reoxidised End products Enzymes involved MAMMALS Hydrogen acceptor Is carbon dioxide produced Is ATP produced Is NAD reoxidised YEAST MAMMALS Ethanol Pyruvate Yes No Yes 2x in glycolysis Yes 2x in glycolysis Yes Yes End products Ethanol and carbon dioxide Lactate Enzymes involved Pyruvate decarboxylase/ ethanol dehydrogenase Lactate dehydrogenase Diving mammals, such as this Galapagos sea lion, evolved adaptations allowing them to stay underwater for prolonged periods of time Suggest what they could be..apply your learning! What physical adaptations? Lower metabolic rate: Diving mammals will slow their heart rate, stop their breathing, and shunt blood flow from their extremities to the brain, heart, and muscles when starting a dive Diving mammals—including whales, seals and otters, have more haemoglobin/myoglobin (oxygen store) in their muscles. Haemoglobin has a higher affinity for oxygen Respiration: large supplies of NAD, so more glycolysis, less build up of lactate; more pH buffers Examination 1. 2. 3. 4. questions! Organisms require energy..(4) (b) In anaerobic conditions compound F.. (5) (b) Yeast cells carry out anaerobic respiration (4) (c) If oxygen is not present (4) 17 marks… When is the majority of ATP produced in respiration??? HOW? What conclusion can you make about the best respiratory substrate? Oxidative phosphorylation Protons flow through channels associated with ATP synthase on inner mitochondrial membrane The more protons=more ATP produced! So more protons in respiratory substrate= more TP when the substrate is respired More H atoms per mole of substrate= more oxygen (final acceptor) needed to respire the substrate! Define the term respiratory substrate; Explain the difference in relative energy values of carbohydrate, lipid and protein So define …… A respiratory substrate is an organic substance that can be used for respiration How many hydrogens! Glucose (C6H12O6) C6H2OH H C1 O H C2 OH HO C3 H H C4 OH H C5 OH H C6 OH H Chain form C5 H O H H C4 OH C1 OH C3 H Ring Chain H C2 OH OH • • • • • • • Glucose is the most common substrate for most mammalian cells Animals store glucose as glycogen, and plants store it as starch Theoretical maximum energy yield for one mole of glucose is 2870 kJ It takes 30.6 kJ to produce 1 mol ATP Respiration of 1 mol glucose should produce nearly 94 mol ATP, but the actual yield is more like 30, as it has an efficiency of 32% Remaining energy used to generate heat Maintains suitable temperature for all the enzyme controlled reactions Draw the amino acid glycine R=H How many hydrogens?? H O H N C C OH H H • • • • • • Excess amino acids are deaminated (removal of amine group converted to urea) Rest is changed to glycogen or fat During fasting/starvation /prolonged exercise: Protein is then hydrolysed (split with water) to amino acids which can be respired Some can be converted to pyruvate, or acetate and then is carried to Krebs cycle Some can enter Krebs directly Number of hydrogen atoms per mole accepted by NAD then used in electron transport chain is slightly more than the number of hydrogen atoms per mole of glucose, so proteins release slightly more energy than equivalent masses of glucose Pyruvate Acetate Directly Draw a glycerol molecule How many hydrogen's???? Draw a triglyceride Lipids are formed by joining fatty acids to a glycerol backbone. Glycerol molecule is same in all fats, so it is the fatty acid that gives fats their different properties O H H C OH H C OH H C OH H HO C O C17H35 HO C O C17H35 HO C C17H35 CARBOXYL GROUP (acid) Condensation reaction: Acid group on fatty acid Glycerol (C3H8O3) + OH Hydroxyl group of glycerol =Covalent bond= ester bond Palmitic acid: a fatty acid • • • • • Made of fatty acids and glycerol Glycerol can be converted to glucose, fatty acids can’t Contain many carbon atoms and hydrogen atoms SO SOURCE OF MANY PROTONS= Much ATP! What happens to the fatty acids?? • • • • • Fatty acids combined with CoA after ATP is used to hydrolyse (split using water) to AMP (adenosine monophosphate) + 2 Pi Fatty acid CoA complex taken to matrix and broken down into 2- carbon acetyl groups that are attached to CoA Reduced NAD and FAD are formed Acetyl groups are released from CoA and enter Krebs producing 3 NADH, 1 FADH and 1 ATP (slp) NADH can then go to electron transport chain • ATP produced by chemiosmosis Coenzyme A Fatty Acid Fatty Acid Coenzyme A complex NAD + FAD Reduced NAD + FAD Many 2-carbon acetyl groups 2-carbon acetyl groups go to the Krebs Cycle Coenzyme A How the respiratory substrates are used Glycogen or starch Protein Glucose Lipid Amino Acids Pyruvate fatty Acids Acetylcoenzyme A Krebs cycle Respiratory Substrate Carbohydrate Lipid Protein Guess?? Mean energy value/kJ g-1 Respiratory Substrate Mean energy value/kJ g-1 Carbohydrate 15.8 Lipid 39.4 Protein 17.0 Key terms mix and match Individually 5 minutes Pairs 5 minutes Table groups WWW/EBI Examination 1. 2. 3. 4. questions! Organisms require energy..(4) (b) In anaerobic conditions compound F.. (5) (b) Yeast cells carry out anaerobic respiration (4) (c) If oxygen is not present (4) 17 marks… Your tasks Using LO sheet: what have we covered in class? Get notes up to date Complete exam question pack TEST.. Left to do respirometer practical Next week: recap lesson, practical lesson, test the next Tuesday (24th March)
































![fermentation[1].](http://s1.studyres.com/store/data/008290469_1-3a25eae6a4ca657233c4e21cf2e1a1bb-150x150.png)


