* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download protein
Clinical neurochemistry wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Paracrine signalling wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Expression vector wikipedia , lookup
Magnesium transporter wikipedia , lookup
Point mutation wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Genetic code wikipedia , lookup
Gene expression wikipedia , lookup
Biochemistry wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Interactome wikipedia , lookup
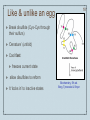
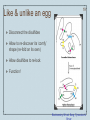
Metalloprotein wikipedia , lookup
Western blot wikipedia , lookup
Protein purification wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
1 Ooopsies & Schedule ❖ ❖ I posted Periodic Table w/ ‘normal’ deadline instead of intended of 9-12 @ 10 p.m. ❖ Kudos to all of you who did it by the posted deadline!! ❖ But for the sake of fairness, deadline now 9-16 Next round: Read paper this weekend ❖ Assessor on Monday/due Thurs p.m. Quiz custodiet ipsos custodes? 2 Who builds the builder? ❖ Machines are 3D ‘folded’* strings of amino acids: ‘proteins’ ❖ What forms (folds) the machines out of strings? ❖ Does anybody see a problem with such a solution? *Folding is the correct term for an amino acid string being brought into correct 3D shape 3 4 What do to with my string of blobs? Beads on a string ❖ It is plausible that a string of distinct, shaped ‘feels’ would self-assemble: Prof. Nowicki’s example ❖ Potential example of amino acid ‘beads’ on a protein ‘string’ shown 5 http://sun.menloschool.org/~dspence/biology/chapter3/chapt3_13.ht 6 Say hello to a special friend ❖ Next week in lab, you’ll revel in the glory of histidine ❖ Today’s special pal: cysteine (recall from Easter Egg Hunt) ❖ Concept: the 20 amino acids are a collections of generalist pieces & specialists with unique capabilities! Sulfur + Sulfur = SulfurSulfur oxidize 7 On the importance of bonding ‘H’ 8 On the importance of bonding ‘H’ (+) (-) 9 10 alpha helix Details, details 11 One, two, three, four Steps in assembling a Big Machine 12 13 Great experiments Boiling an egg, writ small: Scrambled ribonuclease The argument ❖ It is plausible that a string of distinct, shaped ‘feels’ would self-assemble ❖ alpha-helices, beta-sheets can be drawn; pairings postulated ❖ That’s different from evidence 14 How beads organize a string: http://sun.menloschool.org/~dspence/biology/chapter3/chapt3_13.ht ml What it is, is... http://upload.wikimedia.org/wikipedia/commons/c/c0/Ribonuclease_A_7rsa.png 15 Ribonuclease the right way Cartoon of previous ❖ In a protein chain (a.k.a. polypeptide), nearby sulfur sidechains may be covalently joined http://guweb2.gonzaga.edu/faculty/cronk/biochem/images/disulfide_bond_formation.g 16 What they then knew ❖ ‘it cuts up RNA’ * ❖ we can tell if RNA intact vs. cut up ❖ (heat drives proteins wild) ❖ (so do some chemicals--like urea. From the name, guess where we can find that?) 17 *nomenclature moment: ‘enzyme’ = hastener protein that does this: -ase 18 Like & unlike an egg ❖ Break disulfide (Cys-Cys through their sulfurs) ❖ ‘Denature’ (unfold) ❖ Cool fast ❖ ❖ ❖ freezes current state allow disulfides to reform It ‘locks in’ to inactive states Biochemstry, 5th ed. Berg, Tymoczko & Stryer Like & unlike an egg ❖ Disconnect the disulfides ❖ Allow to re-discover its ‘comfy’ shape (re-fold on its own) ❖ Allow disulfides to re-lock ❖ Function! 19 Biochemstry, 5th ed. Berg, Tymoczko & Finding yourself… protein style 20 21 What’s going on in there? Techniques for seeing that which cannot be seen 22 From the lab ❖ By detection of different smells, you deduced ❖ The presence of molecules ❖ Their flightiness ❖ Their structural distinctness ❖ Your possession of receptors & pathways An important idea ❖ To understand a paper, you don’t necessarily need to understand every component ❖ If I tell you that… ❖ ❖ amount of habooxiebooble directly reflects the number of hydrophobic residues in a protein ❖ Protein A has a greater habooxiebooble than Protein B What can you tell me? 23 How can we know the shape of a protein? ❖ X-ray crystallography: the ultimate in (frozen) truth ❖ Circular dichroism (rotation of polarized light) ❖ All you need to know: it happens, and it happens different for different shapes [conformations] of same 1˚ sequence ❖ Cysteine locking ❖ Tyrosine wiggling 24 25 Prions: protein folding diseases Giving a protein a choice isn’t always a good idea 26 What is life? ❖ Replicates ❖ Instructs Mystery diagnosis ❖ Diseases with LOOOONG onset times--years or decade+ ❖ Infectious particles resistant to UV treatments that blow DNA, RNA out of the water Kuru is an incurable degenerative neurological disorder (brain disease) that is a type of transmissible spongiform encephalopathy, caused by a prion found in humans.[1] The term "kuru" derives from the Fore word "kuria/guria", 'to shake'.[2], a reference to the body tremors that are a classic symptom of the disease; it is also known among the Fore as the laughing sickness due to the pathologic bursts of laughter people would display when afflicted with the disease. It is now widely accepted that Kuru was transmitted among members of the Fore tribe of Papua New Guinea via cannibalism.[3] http://en.wikipedia.org/wiki/Kuru_(disease) 27 What’s a ‘prion’? ❖ ❖ 28 Alas ‘ protein infectious particle’ ❖ !?! How can this be? ❖ I will later argue that genetic material must be ‘base like’ with rigid presentation of binary information ❖ Proteins do not qualify! How can they direct reproduction? We may talk viruses & such much later, but this is specifically a protein folding issue, so it’s here Deadly alternatives 29 The secret: conversion of preexisting material to the dark side; not de novo creation Still, this very much blurs the distinction about which structures can & cannot instruct Shown is a small section of a much larger protein--but this is the source of the trouble Biologic Science, Scott Freeman, Fig. 3.15 But wait! There’s more... ❖ Note that besides the bonds between the two, each offers donors & acceptors to the outside ❖ Imagine this going on and on and on... ❖ One ‘prionized’ protein converts another, and they convert others... 30 31 Prion diseases: FYI ❖ http://www.cdc.gov/ncidod/dvrd/prions/ Homework ❖ Assessor drops on Mon. ❖ I challenge you to read on your own 1st ❖ 32 Vocal linked from homepage ContCent => Resources =>Words, Refs & Writing => References & Writing