* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download video slide - Wild about Bio
Survey
Document related concepts
Fatty acid metabolism wikipedia , lookup
Butyric acid wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Phosphorylation wikipedia , lookup
Mitochondrion wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Transcript
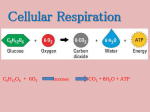
Chapter 9 Cellular Respiration: Harvesting Chemical Energy Energy flows into an ecosystem as sunlight and leaves as heat Photosynthesis generates O2 and organic molecules, which are used in cellular respiration Cellular respiration: Cells use chemical energy stored in organic molecules to regenerate ATP, which powers work Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-2 Light energy ECOSYSTEM Photosynthesis in chloroplasts CO2 + H2O Organic +O molecules 2 Cellular respiration in mitochondria ATP ATP powers most cellular work Heat energy Catabolic Pathways and Production of ATP Fermentation is a partial degradation of sugars that occurs without O2 (no oxygen) Aerobic respiration consumes organic molecules and O2 and yields ATP (requires O2) Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 (does not require oxygen) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Cellular respiration includes both aerobic and anaerobic respiration but is often used to refer to aerobic respiration C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy (ATP + heat) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Redox Reactions: Oxidation and Reduction The transfer of electrons during chemical reactions releases energy stored in organic molecules This released energy is ultimately used to synthesize ATP Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Principle of Redox In oxidation, a substance loses electrons, or is oxidized - The electron donor is called the reducing agent In reduction, a substance gains electrons, or is reduced - The electron receptor is called the oxidizing agent Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings becomes oxidized (loses electron) becomes reduced (gains electron) becomes oxidized becomes reduced The Stages of Cellular Respiration: A Preview Cellular respiration has three stages: Glycolysis (breaks down glucose into two molecules of pyruvate) The citric acid cycle (completes the breakdown of glucose) Oxidative phosphorylation (accounts for most of the ATP synthesis) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-6-3 Electrons carried via NADH and FADH2 Electrons carried via NADH Citric acid cycle Glycolysis Pyruvate Glucose Oxidative phosphorylation: electron transport and chemiosmosis Mitochondrion Cytosol ATP ATP ATP Substrate-level phosphorylation Substrate-level phosphorylation Oxidative phosphorylation The process that generates most of the ATP is called oxidative phosphorylation because it is powered by redox reactions Oxidative phosphorylation accounts for almost 90% of the ATP generated by cellular respiration Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Glycolysis Glycolysis (“splitting of sugar”) breaks down glucose into two molecules of pyruvate Glycolysis occurs in the cytoplasm and has two major phases: Energy investment phase (input 2 ATP) Energy payoff phase (produces 4 ATP) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-8 Energy investment phase Glucose 2 ADP + 2 P 2 ATP used 4 ATP formed Energy payoff phase 4 ADP + 4 P 2 NAD+ + 4 e– + 4 H+ 2 NADH + 2 H+ 2 Pyruvate + 2 H2O Net Glucose 4 ATP formed – 2 ATP used 2 NAD+ + 4 e– + 4 H+ 2 Pyruvate + 2 H2O 2 ATP 2 NADH + 2 H+ The citric acid cycle (Kreb Cycle) In the presence of O2, pyruvate enters the mitochondrion (matrix) Pyruvate must be converted to acetyl CoA, which links the cycle to glycolysis Generates 1 ATP, 3 NADH, and 1 FADH2 per turn The NADH and FADH2 produced by the cycle relay electrons extracted from food to the Animation: Mitochondria electron transport chain Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-10 CYTOSOL MITOCHONDRION NAD+ NADH + H+ 2 1 Pyruvate Transport protein 3 CO2 Coenzyme A Acetyl CoA Fig. 9-11 Pyruvate CO2 NAD+ CoA NADH + H+ Acetyl CoA CoA CoA Citric acid cycle FADH2 2 CO2 3 NAD+ 3 NADH FAD + 3 H+ ADP + P i ATP The Pathway of Electron Transport The electron transport chain is in the cristae of the mitochondrion NADH and FADH2 donate electrons to the electron transport chain Electrons drop in free energy as they go down the chain and are finally passed to O2, forming H2O Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Electrons are passed through a number of proteins The electron transport chain generates no ATP The chain’s function is to break the large freeenergy drop from food to O2 into smaller steps that release energy in manageable amounts Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space H+ then moves back across the membrane, passing through channels in ATP synthase VCAC: Cellular Processes: Electron Transport Chain: First Look VCAC: Cellular Processes: ATP Synthase: The MovieTransport Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-14 INTERMEMBRANE SPACE H+ Stator Rotor Internal rod Catalytic knob ADP + P i ATP MITOCHONDRIAL MATRIX ATP Production by Cellular Respiration Glycolysis- 2 ATP Citric Acid cycle- 2 ATP ATP Synthase- 32 or 34 Total- 38 ATP Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fermentation and anaerobic respiration • • Most cellular respiration requires O2 to produce ATP Glycolysis can produce ATP with or without O2 (in aerobic or anaerobic conditions) In the absence of O2, glycolysis couples with fermentation or anaerobic respiration to produce ATP Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Types of Fermentation Two common types are alcohol fermentation and lactic acid fermentation In alcohol fermentation pyruvate is converted to ethanol Yeast - in brewing, winemaking, and baking Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings In lactic acid fermentation, lactate is the end product Fungi and bacteria - make cheese and yogurt Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Fig. 9-19 Glucose CYTOSOL Glycolysis Pyruvate No O2 present: Fermentation O2 present: Aerobic cellular respiration MITOCHONDRION Ethanol or lactate Acetyl CoA Citric acid cycle