* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Matrix-assisted laser desorption/ionization wikipedia , lookup
Gene expression wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Expression vector wikipedia , lookup
Magnesium transporter wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Peptide synthesis wikipedia , lookup
Interactome wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Point mutation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Western blot wikipedia , lookup
Genetic code wikipedia , lookup
Biosynthesis wikipedia , lookup
Protein purification wikipedia , lookup
Metalloprotein wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
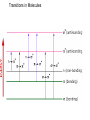
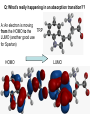
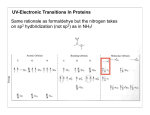
Absorption Spectroscopy See you on the Dark Side of Biochemistry Q: Why is something the color that it is??? Transitions in Molecules Transitions in Some Chomophores QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Conjugated Chromophores Conjugated Chromophores- the more there are the more colorful! QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Conjugated Chromophores- the more there are the more colorful! More conjugation brings excited and ground states closer together Q: What’s really happening in an absorption transition?? A: An electron is moving TRP from the HOMO to the LUMO (another good use for Spartan) HOMO LUMO Uses of Absorbance Spectra: Quantitating Protein by Amino Acid absorption Only Trp, tyr and cys absorb much outside the far UV. Phe is trivial. Uses of Absorbance Spectra: Quantitating Protein by Amino Acid absorption Beer-Lambert (Beer’s) Law Absorbance, A= e lc = Log (1/T) Molar extinction coefficient e has units of M-1 cm-1 and is a constant of proportionality that relates the absorption of molar solutions Mass extinction coefficient e 1% refers to the absorbance of a 1% by mass solution. Uses of Absorbance Spectra: Quantitating Protein by Amino Acid absorption Why not just weigh the protein? * Most samples are typically quantities of milligrams or even micrograms, not grams, and thus, it is difficult to transfer and measure such small amounts * Water is present in proteins, and it is extremely difficult to remove all the water (some water molecules hydrogen bond extremely tightly to proteins). Thus, the mass measurement would include some waters, and would increase the apparent mass of the protein Molar Absorbance of Amino Acid side chains Example: Bovine insulin contains 4 Tyr residues, 6 Cys residues and 0 Trp residues. We can determine the expected molar extinction coefficient at 280nm, e280nm, by the following calculation: e 280nm = (0)(5690) + (4)(1280) + (6)(120) e280nm = 5840 M-1 cm-1 Thus, a 1.0M solution of pure bovine insulin would give an absorbance of 5,840 units at 280nm (obviously, it would have to be diluted considerably to be read accurately since an A > 1.5 units is considered inaccurate). A useful expression relating the parameters of e, concentration (c) and A are derived from the BeerLambert law (assuming 1 cm path length): A/ e280nm = c For example, if a sample of bovine insulin was observed to give an absorbance at 280nm of 0.745 we could calculate the concentration to be: 0.745/5840 M-1 cm-1 = c C = 1.28 x 10-4 M (note: cm-1 drops out with 1 cm pathlength) While this method is generally more accurate than routine “Protein Assays” using colorimetric methods, it is still an approximation and amino acid absorption can be considerably altered by the local environment in the protein. There is a web site ProtParam, http://ca.expasy.org/tools/protparam.htmlthat can be used to estimate protein extinction coefficients, MW and pIs for a given amino acid sequence. Peptide/Protein Absorbance: Once a peptide is formed, any combination of amino acids will absorb strongly around 190-220nm due to the amide. This is why we detect proteins on the HPLC at 210-220 nm and it doesn’t even matter whether they have trp, cys or tyr. Generic Protein Absorption Instrumentation: Scanning Spectrophotometer • Typical Instrumentation: Diode Array Spectrophotometer • Typical