* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES
Gel electrophoresis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Genetic code wikipedia , lookup
Signal transduction wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Biosynthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Proteolysis wikipedia , lookup
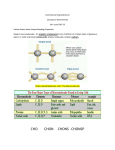
CHAPTER 5 THE STRUCTURE AND FUNCTION OF MACROMOLECULES Chapter 5 Objectives After reading this chapter, completing the study guide, and participating in class, you should be able to: 1. List the levels of biological organization from subatomic particles through macromolecules 2. Describe the distinguishing characteristics of carbohydrates and explain their classification 3. Describe the important biological functions of polysaccharides 4. Explain what distinguishes lipids from other classes of biological macromolecules 5. Describe the unique properties, building blocks and biological roles of fats, phospholipids and steroids 6. Distinguish proteins from the other classes of macromolecules and list the biological functions which members of this class perform 7. List and be able to recognize the four major components of a typical amino acid and explain how amino acids may be grouped according to the nature of their side chain 8. Identify a peptide bond and describe how it is formed 9. Explain what determines protein conformation and why it is important 10. Name the four levels of protein structure and briefly describe from what aspect of protein structure each is derived 11. Define denaturation and explain how proteins may be denatured 12. Describe the characteristics that distinguish nucleic acids from the other classes of macromolecules 13. Summarize the functions of nucleic acids 14. Juggle three flaming batons...just checking that you're still paying attention (smile!) 15. List the major components of a nucleotide and describe how these monomers are linked together to form a nucleic acid 16. Distinguish between a pyrimidine and a purine and name those which occur in nucleic acids. 17. Describe at least one function of nucleotides other than their inclusion in nucleic acids 18. Briefly describe the three-dimensional structure of DNA Introduction • Cells join smaller organic molecules together to form larger molecules. • These larger molecules, macromolecules, may be composed of thousands of atoms and weigh over 100,000 daltons. • The four major classes of macromolecules are: carbohydrates, lipids, proteins, and nucleic acids. Polymer Key Principles 1. Most macromolecules are polymers 2. An immense variety of polymers can be built from a small set of monomers • Pgs. 58-60 1. Most macromolecules are polymers • Three of the four classes of macromolecules form chainlike molecules called polymers (greek poly = many, mer = parts). – Polymers consist of many similar or identical building blocks linked by covalent bonds. • The repeated units are small molecules called monomers (mono = one). – Some monomers have other functions of their own. Making or Breaking Polymers • The chemical mechanisms that cells use to make and break polymers are similar for all classes of macromolecules. • See figure 5.2, pg 59 Making Polymers • Monomers are connected by covalent bonds via a condensation reaction or dehydration reaction. – One monomer provides a hydroxyl group and the other provides a hydrogen and together these form water. – This process requires energy and is aided by enzymes. Breaking Down Polymers • The covalent bonds connecting monomers in a polymer are disassembled by hydrolysis. – In hydrolysis as the covalent bond is broken a hydrogen atom and hydroxyl group from a split water molecule attaches where the covalent bond used to be. – Hydrolysis reactions dominate the digestive process, guided by specific enzymes. 2. An immense variety of polymers can be built from a small set of monomers • Each cell has thousands of different macromolecules. – These molecules vary among cells of the same individual, even more among unrelated individuals of a species, and are even greater between species. • This diversity comes from various combinations of the 40-50 common monomers and other rarer ones. – These monomers can be connected in various combinations like the 26 letters in the alphabet can be used to create a great diversity of words. – Biological molecules are even more diverse. Objective 1 • List the levels of biological organization from subatomic particles through macromolecules