* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Compounds The Big Four
Citric acid cycle wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Magnesium transporter wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Interactome wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Point mutation wikipedia , lookup
Peptide synthesis wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Western blot wikipedia , lookup
Metalloprotein wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
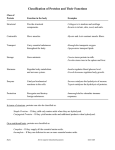
Organic Compounds The Big Four Objectives: - Highlight the similarities between protein, nucleic acid, lipids and carbohydrates - Examine amino acid structure and amino acid chains - Determine the function and shape of proteins and why they are Mr. Swift’s favourite. What are the Big Four? • The big four refer to the four organic compounds found in living things. They include: – Proteins – Nucleic Acids – Lipids – Carbohydrates Similarities in the Big Four • They all have carbon as their core structure • Contain hydrogen, oxygen and several other atoms. • They are called macromolecules (means giant molecules) and are made from thousands of smaller molecules. • The smaller units are called monomers and they join together to form polymers. Polymer Monomer • Protein • Amino acids • Nucleic Acids • Nucleotides • Lipids • Glycerol and Fatty Acids • Carbohydrates • Glucose Proteins • Proteins have amino acids as their basic structure • Only 20 amino acids • Amino acids are all similar in structure. They all have: 1. 2. 3. 4. 5. Central carbon atom Carboxylic acid group Amine group Single hydrogen atom R group • An R group is an arrangement of different atoms Proteins Drawing an Amino Acid Proteins Glycine Proteins • Circle the two you want to memorize • Star methionine Proteins • • • • Dehydration Synthesis Dehydration refers to a loss of water When one amino acid joins to another, the carboxyl group of the first bonds to the amine group of the second. This produces a peptide bond. Water is released in this process. Proteins Glycine + Glycine → Gly-Gly + H2O The reverse of this reaction is called hydrolysis Proteins • Draw the dehydration synthesis of 2 alanine molecules. Proteins • 2 amino acids are a dipeptide molecule. • More than 2 amino acids are a polypeptide • More than 200 amino acids are a protein. Proteins • Draw the dehydration synthesis of an alanine and a glycine molecule. Functions of Proteins • • • • • Structural molecule Enzymes Hormones Antibodies Passive and active channels in the plasma membrane Protein Shapes • Primary (Linear) – the sequence of amino acids are in a protein chain. Protein Shapes • Secondary (folded or helical) – the amino acids in the chain are twisted or folded upon themselves. Protein Shapes • Tertiary (3D shape) – the chain itself is folded because of the interactions between the amino acids with large R groups. Protein Shapes • Quarternary – multiple polypeptides all with a 3D shape. Protein Video • http://www.youtube.com/watch?v=wctkPUUpUc Activity • Building a quaternary protein structure • Notice that if you press or stretch your protein gently it springs back into place. Proteins are somewhat flexible • If you stretch or press it harder the protein losses its shape and is unable to return to it’s original configuration. This can happen to a protein that had been altered by heat or chemical denaturation. Review • • • • • • • • • • All organic compounds have __________ as their core element. Large molecules made up of repeating units are called __________. The building blocks of organic compounds are called __________. All 20 amino acids have the same structural blueprint; a central __________, an __________ group, a __________ acid group, a single ___________ and an _____________. The simplest amino acid is called ___________. Amino acids link together in a process called ________________. Amino acids are linked by a special covalent bond called a ____________. The first amino acid set down in every protein is _____________. Five important functions of my favorite organic compound are _____________, _________________, ________________, ________________, and ______________________________. Protein are found in 4 shapes: ________________, ___________________, ______________________ and _____________________. Review • • • • • • • • • • All organic compounds have Carbon as their core element. Large molecules made up of repeating units are called Polymers. The building blocks of organic compounds are called Monomers. All 20 amino acids have the same structural blueprint; a central Carbon, an Amine group, a Carboxyl acid group, a single Hydrogen and an R-group. The simplest amino acid is called Glycine. Amino acids link together in a process called Dehydration Synthesis. Amino acids are linked by a special covalent bond called a Peptide. The first amino acid set down in every protein is Methionine. Five important functions of my favorite organic compound are Structural, Hormones, Enzymes, Antibodies and Carrier Protein. Protein are found in 4 shapes: Primary, Secondary, Tertiary and Quaternary. Factors that Alter the Shape of Protein • Temperature - Protein Structure • Changes in pH – page 51 in your book • Denaturation – temporary change in shape • Coagulation – permanent change in shape Page 51 in the Text book 1. What variable is plotted on the x-axis? – Time in seconds What variable is plotted on the y-axis? - Pressure of oxygen in kPa 2. How did the rate of reaction change over time in the control reaction? – The rate was very rapid and then levelled off 3. Suggest an explanation for the change in the control at about 40 seconds. – The hydrogen peroxide was used up Page 51 in the Text book 4. What effect do acids and bases have on the enzyme catalase? – The base makes it less effective and the acid deactivates it so the reaction cannot take place 5. Would it be valid to conclude that if a base were added to a reaction the rate of the reaction would slow down? – The blue line shows that the pressure of oxygen was lower when the base was added, so yes, this would be a valid conclusion 6. Predict what would happen if vinegar were added to a solution of hydrogen peroxide and catalase. – Since vinegar is an acid, it would most likely make the reaction not take place. Proteins as Enzymes • Enzymes are proteins that act as biological catalysts • Catalysts are substances that speed up the chemical reaction in cells • In chemical reactions the elements or compounds entering into the reaction are called the reactants, the elements or compounds produced by the reaction are called the products. Proteins as Enzymes • Only function in one chemical reaction – specificity (lock and key principle) • Are unaffected by the reaction, so they can be used over again. • If the shape of the enzyme changes, the enzyme can’t do it’s job. • Reduce the activation energy needed to start the reaction Catabolic Reactions Anabolic Reactions Enzymes http://www.learnerstv.com/animation/animation. php?ani=324&cat=biology Review • The big four • Monomers and polymers • All organic compounds have carbon as their core structure • Parts of an amino acid • Dehydration synthesis and hydrolysis • Functions of proteins • Shapes of proteins Review • • • • • Denaturation and coagulation Factors that alter the shape of proteins Reactants and products Specificity Catabolic and anabolic Objectives • - Highlight the similarities between protein, nucleic acid, lipids and carbohydrates • - Examine amino acid structure and amino acid chains • - Determine the function and shape of proteins and why they are Mr. Swift’s favourite.