* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Session 2
Cryobiology wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Proteolysis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Signal transduction wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Biochemical cascade wikipedia , lookup
Photosynthesis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
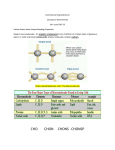
FTCE SAE BIOLOGY PREPARATION COURSE Instructor Valerie Ruwe [email protected] SESSION NORMS No side bars Work on assigned materials only Keep phone on vibrate only If a call must be taken please leave the room to do so SESSION AGENDA Session I: Pre-Test, Competencies 1 & 2 Session II: Competencies 3,4 Session III: Competencies 5,6 Session IV: Competencies 7,8 Session V: Competencies 9,10 3. KNOWLEDGE OF THE CHEMICAL PROCESSES OF LIVING THINGS12 % 1. Identify the structures, functions, and importance of inorganic and organic compounds (e.g., water, mineral salts, carbohydrates, lipids, proteins, nucleic acids) in cells 2. Compare and apply the laws of thermodynamics to living systems, including the role of enzymes in biological reactions 3. Predict the effects of changes in pH, temperature, substrate concentration, and enzyme concentration on enzyme activity 4. Identify substrates, products, and relationships between glycolysis, Krebs cycle, and electron transport, including the respiration of carbohydrates, fats, and amino acids 5. Compare end products and energy yields of alcoholic fermentation, lactic acid fermentation, and aerobic respiration. 6. Identify the raw materials and products of C-3 photosynthesis, including the Calvin cycle, light dependent and light independent reactions, and factors that affect their rate 7. Identify key differences between C-3, C-4, and CAM photosynthesis, and the ecological significance of these pathways 3. KNOWLEDGE OF THE CHEMICAL PROCESSES OF LIVING THINGS12 % 8. Identify and analyze the process of chemiosmosis in photosynthesis and respiration 9. Compare heterotrophy and autotrophy and the roles of these processes in the environment 10. Define antigen and antibody and recognize the antigen-antibody reaction 11. Compare active and passive immunity, identifying the positive and negative effects of vaccines and inoculations 12. Evaluate the roles of cell recognition (e.g., cell-to-cell signaling, autoimmune diseases, tissue rejection, cancer, pollen/stigma-style interaction) in normal and abnormal cell activity 13. Identify the effect of environmental factors on the biochemistry of living things (e.g., UV light effects on melanin and vitamin D production). 14. Identify the roles of ATP and ADP in cellular processes 15. Compare chemosynthetic and photosynthetic processes and the roles of organisms using these processes in the ecosystem 16. Identify cell-to-cell communication in living things (e.g., electrical, molecular, ionic) IDENTIFY THE STRUCTURES, FUNCTIONS, AND IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS Water in Living Things Ice Floats Water is a solvent Water has high specific heat capacity Water has high heat of vaporization Evaporative Cooling Cohesion Adhesion Water held together by polar covalent bond Between water molecules hydrogen bonding. IDENTIFY THE STRUCTURES, FUNCTIONS, AND IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS Minerals Inorganic Compounds Cofactors for enzymes Vitamins Organic Compounds Coenymes for enzymes IDENTIFY THE STRUCTURES, FUNCTIONS, AND IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS Monomers are small molecules which may be joined together in a repeating fashion to form more complex molecules called polymers. A polymer may be a natural or synthetic macromolecule comprised of repeating units of a smaller molecule (monomers). Dehydration (Condensation) A process of linking monomers, called dehydration condensation, involves the removal of two hydrogen atoms and one oxygen atom to form water. One way this might happen is where several generic monomers are shown with -OH groups that could be used for linking. Hydrolysis A process of breaking down polymers, called hydrolysis, involves the insertion of water. IDENTIFY THE STRUCTURES, FUNCTIONS, AND IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS Carbohydrates are organic compounds made of carbon, hydrogen, and oxygen atoms in the proportion of 1:2:1. The building blocks of carbohydrates are single sugars, called monosaccharides, such as glucose, C6H12O6, and fructose. Disaccharides are double sugars formed when two monosaccharides are joined Polysaccharides such as starch,are chains of three or more monosaccharide's. Glysocidc Linkage IDENTIFY THE STRUCTURES, FUNCTIONS, AND IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS Lipids are nonpolar molecules that are not soluble in water. They include fats, phospholipids, steroids, and waxes. Fats are lipids that store energy. Triglyceride: A typical fat contains three fatty acids bonded to a glycerol molecule backbone. Ester Bonds Phospholipids Head: phosphate group (choline) which is hydrophilic Tail: two fatty acids which are hydrophobic Make Up Cell Membrane IDENTIFY THE STRUCTURES, FUNCTIONS, AND IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS Proteins A protein is a large molecule formed by linked smaller molecules called amino acids. Amino acids are the building blocks of proteins. Twenty different amino acids are found in proteins. Buiret test runs lilac when positive for protein. Peptide Bond: between amino & carboxylic group Unit 1 STRUCTURE OF PROTEINS IMPORTANCE OF INORGANIC AND ORGANIC COMPOUNDS (E.G., WATER, MINERAL SALTS, CARBOHYDRATES, LIPIDS, PROTEINS, NUCLEIC ACIDS) IN CELLS Nucleic Acids There are two types of nucleic acids—DNA and RNA—and each type contains four kinds of nucleotides. DNA, or deoxyribonucleic acid, consists of two strands of nucleotides that spiral around each other. RNA, or ribonucleic acid, consists of a single strand of nucleotides. Nucleotide: phosphate group + Pentose Sugar + nitrogenous base Pentose Sugar: DNA (deoxyribose) & RNA(ribose) Nitrogenous Base Purines (Double Rings) Adenine & Guanine Pyrminidines (Single Rings) Cytosine, Thymine, & Uracil Unit 1 Section Chemistry of Cells STRUCTURE OF NUCLEIC ACIDS IDENTIFY THE ROLES OF ATP AND ADP IN CELLULAR PROCESSES ATP or adenosine triphosphate, is a single nucleotide with two extra energy-storing phosphate groups. When food molecules are broken down inside cells, some of the energy in the molecules is stored temporarily in ATP. IDENTIFY THE ROLES OF ATP AND ADP IN CELLULAR PROCESSES COMPARE AND APPLY THE LAWS OF THERMODYNAMICS TO LIVING SYSTEMS, INCLUDING THE ROLE OF ENZYMES IN BIOLOGICAL REACTIONS COMPARE AND APPLY THE LAWS OF THERMODYNAMICS TO LIVING SYSTEMS, INCLUDING THE ROLE OF ENZYMES IN BIOLOGICAL REACTIONS The energy needed to start a chemical reaction is called activation energy. Even in a chemical reaction that releases energy, activation energy must be supplied before the reaction can occur. Enzymes are substances that increase the speed of chemical reactions. Most enzymes are proteins. Enzymes are catalysts, which are substances that reduce the activation energy of a chemical reaction. Substrate specific COMPARE AND APPLY THE LAWS OF THERMODYNAMICS TO LIVING SYSTEMS, INCLUDING THE ROLE OF ENZYMES IN BIOLOGICAL REACTIONS PREDICT THE EFFECTS OF CHANGES IN PH, TEMPERATURE, SUBSTRATE CONCENTRATION, AND ENZYME CONCENTRATION ON ENZYME ACTIVITY Temperature and pH value can alter an enzymes effectiveness. The enzymes that are active at any one time in a cell determine what happens in that cell. An enzyme’s shape determines its activity. Typically, an enzyme is a large protein with one or more deep folds on its surface. These folds form pockets called active sites. Enzyme inhibitors: are molecules that interact in some way with the enzyme to prevent it from working in the normal manner. Non-specific methods of inhibition include any physical or chemical changes which ultimately denatures the protein portion of the enzyme and are therefore irreversible. COMPARE HETEROTROPHY AND AUTOTROPHY AND THE ROLES OF THESE PROCESSES IN THE ENVIRONMENT Temperature and pH value can alter an enzymes Directly or indirectly, almost all of the energy in living systems needed for metabolism comes from the sun. Metabolism involves either using energy to build molecules or breaking down molecules in which energy is stored. Photosynthesis is the process by which light energy is converted to chemical energy. Organisms that use energy from sunlight or from chemical bonds in inorganic substances to make organic compounds are called autotrophs. The chemical energy in organic compounds can be transferred to other organic compounds or to organisms that consume food. Organisms that must get energy from food instead of directly from sunlight or inorganic substances are called Heterotrophs. Cellular respiration is a metabolic process similar to burning fuel. COMPARE CHEMOSYNTHETIC AND PHOTOSYNTHETIC PROCESSES AND THE ROLES OF ORGANISMS USING THESE PROCESSES IN THE ECOSYSTEM Ecosystems depend upon the ability of some organisms to convert inorganic compounds into food that other organisms can then exploit. In most cases, primary food production occurs in a process called photosynthesis, which is powered by sunlight. In a few environments, primary production happens though a process called chemosynthesis, which runs on chemical energy. Together, photosynthesis and chemosynthesis fuel all life on Earth. The diagram below compares examples of these two processes chemosynthesis in a seafloor hydrothermal vent bacterium, and photosynthesis in a terrestrial plant. IDENTIFY THE RAW MATERIALS AND PRODUCTS OF C-3 PHOTOSYNTHESIS, INCLUDING THE CALVIN CYCLE, LIGHT DEPENDENT AND LIGHT INDEPENDENT REACTIONS, AND FACTORS THAT AFFECT THEIR RATE The Stages of Photosynthesis Stage 1 Energy is captured from sunlight. Stage 2 Light energy is converted to chemical energy, which is temporarily stored in ATP and the energy carrier molecule NADPH. Stage 3 The chemical energy stored in ATP and NADPH powers the formation of organic compounds, using carbon dioxide, CO2. IDENTIFY THE RAW MATERIALS AND PRODUCTS OF C-3 PHOTOSYNTHESIS, INCLUDING THE CALVIN CYCLE, LIGHT DEPENDENT AND LIGHT INDEPENDENT REACTIONS, AND FACTORS THAT AFFECT THEIR RATE Chapter 5 ELECTRON TRANSPORT CHAINS OF PHOTOSYNTHESIS IDENTIFY THE RAW MATERIALS AND PRODUCTS OF C-3 PHOTOSYNTHESIS, INCLUDING THE CALVIN CYCLE, LIGHT DEPENDENT AND LIGHT INDEPENDENT REACTIONS, AND FACTORS THAT AFFECT THEIR RATE Factors that Affect Photosynthesis Photosynthesis is directly affected by various environmental factors. In general, the rate of photosynthesis increases as light intensity increases until all the pigments are being used. Photosynthesis is most efficient within a certain range of temperatures. IDENTIFY KEY DIFFERENCES BETWEEN C-3, C-4, AND CAM PHOTOSYNTHESIS, AND THE ECOLOGICAL SIGNIFICANCE OF THESE PATHWAYS IDENTIFY KEY DIFFERENCES BETWEEN C-3, C-4, AND CAM PHOTOSYNTHESIS, AND THE ECOLOGICAL SIGNIFICANCE OF THESE PATHWAYS IDENTIFY SUBSTRATES, PRODUCTS, AND RELATIONSHIPS BETWEEN GLYCOLYSIS, KREBS CYCLE, AND ELECTRON TRANSPORT, INCLUDING THE RESPIRATION OF CARBOHYDRATES, FATS, AND AMINO ACIDS Cellular respiration occurs in two stages: Stage 1 Glucose is converted to pyruvate, producing a small amount of ATP and NADH. Stage 2 When oxygen is present, pyruvate and NADH are used to make a large amount of ATP. When oxygen is not present, pyruvate is converted to either lactate or ethanol and carbon dioxide. Chapter 5 CELLULAR RESPIRATION IDENTIFY SUBSTRATES, PRODUCTS, AND RELATIONSHIPS BETWEEN GLYCOLYSIS, KREBS CYCLE, AND ELECTRON TRANSPORT, INCLUDING THE RESPIRATION OF CARBOHYDRATES, FATS, AND AMINO ACIDS Glycolysis In the first stage of cellular respiration, glucose is broken down in the cytoplasm during a process called glycolysis. As glucose is broken down, some of its hydrogen atoms are transferred to an electron acceptor called NAD+. This forms an electron carrier called NADH. IDENTIFY SUBSTRATES, PRODUCTS, AND RELATIONSHIPS BETWEEN GLYCOLYSIS, KREBS CYCLE, AND ELECTRON TRANSPORT, INCLUDING THE RESPIRATION OF CARBOHYDRATES, FATS, AND AMINO ACIDS Krebs Cycle Acetyl-CoA enters a series of enzyme-assisted reactions called the Krebs cycle, which follows five steps: Step 1 Acetyl-CoA combines with a four-carbon compound, forming a six-carbon compound and releasing coenzyme A. Step 2 Carbon dioxide is released from the six-carbon compound, forming a five-carbon compound. Electrons are transferred to NAD+, making a molecule of NADH. Step 3 Carbon dioxide is released from the compound. A molecule of ATP and a molecule of NADH are made. Step 4 The existing four-carbon compound is converted to a new four-carbon compound. Electrons are transferred to an electron acceptor called FAD, making a molecule of FADH2, another type of electron carrier. Step 5 The new four-carbon compound is then converted to the four-carbon compound that began the cycle. Another molecule of NADH is produced. Chapter 5 KREBS CYCLE IDENTIFY SUBSTRATES, PRODUCTS, AND RELATIONSHIPS BETWEEN GLYCOLYSIS, KREBS CYCLE, AND ELECTRON TRANSPORT, INCLUDING THE RESPIRATION OF CARBOHYDRATES, FATS, AND AMINO ACIDS Electron Transport Chain In aerobic respiration, electrons donated by NADH and FADH2 pass through an electron transport chain. In eukaryotic cells, the electron transport chain is located in the inner membranes of mitochondria. At the end of the electron transport chain, hydrogen ions and spent electrons combine with oxygen molecules forming water molecules. Chapter 5 ELECTRON TRANSPORT CHAIN OF AEROBIC RESPIRATION COMPARE END PRODUCTS AND ENERGY YIELDS OF ALCOHOLIC FERMENTATION, LACTIC ACID FERMENTATION, AND AEROBIC RESPIRATION When oxygen is not present, NAD+ is recycled in another way. Under anaerobic conditions, electrons carried by NADH are transferred to pyruvate produced during glycolysis. This process recycles NAD+ needed to continue making ATP through glycolysis. The recycling of NAD+ using an organic hydrogen acceptor is called fermentation COMPARE END PRODUCTS AND ENERGY YIELDS OF ALCOHOLIC FERMENTATION, LACTIC ACID FERMENTATION, AND AEROBIC RESPIRATION Lactic Acid and Alcoholic Fermentation When oxygen is not present, cells recycle NAD+ through fermentation. IDENTIFY AND ANALYZE THE PROCESS OF CHEMIOSMOSIS IN PHOTOSYNTHESIS AND RESPIRATION IDENTIFY THE EFFECT OF ENVIRONMENTAL FACTORS ON THE BIOCHEMISTRY OF LIVING THINGS (E.G., UV LIGHT EFFECTS ON MELANIN AND VITAMIN D PRODUCTION) Melanocytes are pigment-producing cells in the stratum basale, the deepest layer of skin tissue. Everyone has about the same number of melanocytes; what causes variations in skin color is the amount of melanin, or pigment, these cells produce and how spread out the pigment is from the center of the cell. Also, in darker-skinned people, the melanin breaks down more slowly, and is seen in all the layers of the skin, whereas in fair-skinned people, it breaks down quickly and is rarely seen above the stratum basale. IDENTIFY THE EFFECT OF ENVIRONMENTAL FACTORS ON THE BIOCHEMISTRY OF LIVING THINGS (E.G., UV LIGHT EFFECTS ON MELANIN AND VITAMIN D PRODUCTION) Vitamin D is a fat-soluble vitamin that is naturally present in very few foods, added to others, and available as a dietary supplement. It is also produced endogenously when ultraviolet rays from sunlight strike the skin and trigger vitamin D synthesis. Vitamin D obtained from sun exposure, food, and supplements Vitamin D promotes calcium absorption in the gut and maintains adequate serum calcium and phosphate concentrations to enable normal mineralization of bone IDENTIFY CELL-TO-CELL COMMUNICATION IN LIVING THINGS (E.G., ELECTRICAL, MOLECULAR, IONIC) In multicellular organisms like us, cellto-cell communication is of prime importance for proper development and function of the organisms as a whole. Cells communicate with each other by different means. Adjacent cells can communicate via cell surface molecules or via specific junctions that allow the exchange of solutes or the propagation of changes in membrane potential. Cells that are not in direct contact with each other may communicate via soluble messenger molecules. Once released, a messenger molecule acts on other cells that are responsive to it (target cells). In general, responsiveness requires the presence of specific receptors for the messenger molecule at the target cell. EVALUATE THE ROLES OF CELL RECOGNITION (E.G., CELL-TO-CELL SIGNALING, AUTOIMMUNE DISEASES, TISSUE REJECTION, CANCER, POLLEN/STIGMA-STYLE INTERACTION) IN NORMAL AND ABNORMAL CELL ACTIVITY cell-to-cell recognition (glycoproteins) EVALUATE THE ROLES OF CELL RECOGNITION (E.G., CELL-TOCELL SIGNALING, AUTOIMMUNE DISEASES, TISSUE REJECTION, CANCER, POLLEN/STIGMA-STYLE INTERACTION) IN NORMAL AND ABNORMAL CELL ACTIVITY ny person (or animal) that develops an autoAimmune disease, discovers that a certain part of his body, or many parts, become inflamed and painful. What is happening is that the cells of the Immune System are attacking those cells of the body that the Immune System considers “foreign.” Normally, the cells of the Immune System recognize all the cells of the body and do not consider them “foreign cells.” Normally, no cells of the body are attacked by the Immune System. The Immune System is “trained” by a gland called the Thymus Gland to recognize all the cells of its own body. This “training” is still not completely understood. However, the cells of the Immune System normally recognizes the body’s cells and only attacks “foreign” cells. This extremely delicate situation is changed in people with “Auto-Immune” diseases, and the Immune System attacks specific cells of the body, thereby creating over 80 different diseases. EVALUATE THE ROLES OF CELL RECOGNITION (E.G., CELL-TOCELL SIGNALING, AUTOIMMUNE DISEASES, TISSUE REJECTION, CANCER, POLLEN/STIGMA-STYLE INTERACTION) IN NORMAL AND ABNORMAL CELL ACTIVITY Transplant rejection is a process in which a transplant recipient's immune system attacks the transplanted organ or tissue. These harmful substances have proteins called antigens on their surfaces. As soon as these antigens enter the body, the immune system recognizes them as foreign and attacks them. In the same way, an organ that is not matched can trigger a blood transfusion reaction or transplant rejection. To help prevent this reaction, doctors "type" both the organ donor and the person who is receiving the organ. The more similar the antigens are between the donor and recipient, the less likely that the organ will be rejected. EVALUATE THE ROLES OF CELL RECOGNITION (E.G., CELL-TOCELL SIGNALING, AUTOIMMUNE DISEASES, TISSUE REJECTION, CANCER, POLLEN/STIGMA-STYLE INTERACTION) IN NORMAL AND ABNORMAL CELL ACTIVITY Most antigens expressed by human cancer cells and recognized by host T cells and antibodies are nonmutated self antigens — molecules also expressed on the surface of normal cells. These self antigens are ineffective at triggering immune responses against cancer cells, which provides one explanation for the difficulties in trying to immunize against human cancer. A new study describes how tumors can avoid recognition by the immune system and how enhancing the affinity of the interaction between a self antigen and the MHC-I molecule may lead to cancer immunity. DEFINE ANTIGEN AND ANTIBODY AND RECOGNIZE THE ANTIGENANTIBODY REACTION Antigens are defined as substances recognized by the body as foreign, causing the body to produce an antibody to react specifically with it Factors determining whether an antigen will stimulate an antibody response: Degree of foreignness. Only human blood is transfused to humans. Size and complexity. Although red cells are smaller than white blood cells, they tend to be more antigenic due to the complexity of the antigens on the cell surface. Some are proteins and others are oligosaccharides. Dose of antigen administered. How much antigen is the individual exposed to and what is the frequency of that exposure. Genetic makeup of host may also dictate whether an antibody is produced. Some individuals have a greater ability to make antibody and others have the antigen so they would not make the antibody. Antibody: Proteins produced by lymphocytes as a result of stimulation by an antigen which can then interact specifically with that particular antigen. COMPARE ACTIVE AND PASSIVE IMMUNITY, IDENTIFYING THE POSITIVE AND NEGATIVE EFFECTS OF VACCINES AND INOCULATIONS. Active Immunity - Vaccines are used for health purposes to expose our bodies to a particular antigen. These antigens are usually killed or severely weakened to decrease their potency. After destroying these pathogens, the body stores some T cells as memory cells, due to the fact they code for a particular antigen and can be when needed. This memory in T cells can be a means of artificially acquiring immunity while a genuine attack by a pathogen is a naturally acquired type of immunity. Passive Immunity - This is where immunity to particular antigens as a result of genetic traits passed on from parents rendering the offspring immune to a particular pathogenic threat. COMPARE ACTIVE AND PASSIVE IMMUNITY, IDENTIFYING THE POSITIVE AND NEGATIVE EFFECTS OF VACCINES AND INOCULATIONS. A substance used to stimulate the production of antibodies and provide immunity against one or several diseases, prepared from the causative agent of a disease, its products, or a synthetic substitute, treated to act as an antigen without inducing the disease BREAK TIME!!! 4. KNOWLEDGE OF THE INTERACTION OF CELL STRUCTURE AND FUNCTION 10 % 1. Identify and analyze the major events in the development of the cell theory. 2. Distinguish between the major structural characteristics of prokaryotic and eukaryotic cells. 3. Relate the structure of cell organelles to their functions. 4. dentify and evaluate the events of each phase of the cell cycle (G1, S, G2, M) and the regulatory mechanisms of the cycle. 5. Compare the mechanisms and results of nuclear division (karyokinesis) and cell division (cytokinesis) in plant and animal cells. 6. Compare characteristics of the major taxa (kingdoms/domains), including cellular characteristics. 7. Evaluate the relationships between the structures and functions of cell membrane elements. 8. Identify and compare active and passive transport mechanisms. IDENTIFY AND ANALYZE THE MAJOR EVENTS IN THE DEVELOPMENT OF THE CELL THEORY. All living things are composed of cells Cells are the basic units of structure and function in living things All cells are produced from other cells DISTINGUISH BETWEEN THE MAJOR STRUCTURAL CHARACTERISTICS OF PROKARYOTIC AND EUKARYOTIC CELLS. RELATE THE STRUCTURE OF CELL ORGANELLES TO THEIR FUNCTIONS. IDENTIFY AND EVALUATE THE EVENTS OF EACH PHASE OF THE CELL CYCLE (G1, S, G2, M) AND THE REGULATORY MECHANISMS OF THE CYCLE. There is an independent cell cycle control system made up of proteins that are different from the effector proteins that directly perform mitosis, G1, DNA replication, or G2. Brakes that can stop the cycle at specific checkpoints (a.k.a restriction points) regulate the control system. At checkpoints, feedback signals conveying information about the effector processes, or extracellular signals, can delay progress of the control system itself, so as to prevent it from triggering the next effector process before the previous one is finished. The two major checkpoints occur at G1, just before entry into S phase, and at G2 shortly before mitosis. IDENTIFY AND EVALUATE THE EVENTS OF EACH PHASE OF THE CELL CYCLE (G1, S, G2, M) AND THE REGULATORY MECHANISMS OF THE CYCLE. The proteins involved in regulating cell division events no longer appropriately drive progression from one cell cycle stage to the next. Rather than lacking function, cancer cells reproduce at a rate far beyond the normally tightly regulated boundaries of the cell cycle. Cells that progress through the cell cycle unchecked may eventually form malignant tumors, where masses of cells grow and divide uncontrollably, then develop the ability to spread and migrate throughout the body. COMPARE THE MECHANISMS AND RESULTS OF NUCLEAR DIVISION (KARYOKINESIS) AND CELL DIVISION (CYTOKINESIS) IN PLANT AND ANIMAL CELLS Mitosis is division of the nucleus Interphase, Prophase, Metaphase, Anaphase, Telophase Cytokensis division of the cytoplasm COMPARE THE MECHANISMS AND RESULTS OF NUCLEAR DIVISION (KARYOKINESIS) AND CELL DIVISION (CYTOKINESIS) IN PLANT AND ANIMAL CELLS COMPARE CHARACTERISTICS OF THE MAJOR TAXA (KINGDOMS/DOMAINS) INCLUDING CELLULAR CHARACTERISTICS EVALUATE THE RELATIONSHIPS BETWEEN THE STRUCTURES AND FUNCTIONS OF CELL MEMBRANE ELEMENTS. 1. Channel Proteins - form small openings for molecules to difuse through 2. Carrier Proteins- binding site on protein surface "grabs" certain molecules and pulls them into the cell 3. Receptor Proteins - molecular triggers that set off cell responses (such as release of hormones or opening of channel proteins) 4. Cell Recognition Proteins - ID tags, to idenitfy cells to the body's immune system 5. Enzymatic Proteins - carry out metabolic reactions IDENTIFY AND COMPARE ACTIVE AND PASSIVE TRANSPORT MECHANISMS. •PASSIVE TRANSPORT: •no ATP required; moves from high concentration toward low naturally • • • simple diffusion facilitated diffusion osmosis IDENTIFY AND COMPARE ACTIVE AND PASSIVE TRANSPORT MECHANISMS. ACTIVE TRANSPORT: requires ATP to move items against the concentration gradient from low toward high