* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cholinergic - stjpap 2011
Drug interaction wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
CCR5 receptor antagonist wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Toxicodynamics wikipedia , lookup
NMDA receptor wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
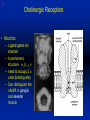
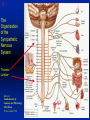
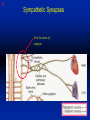
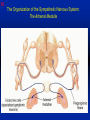
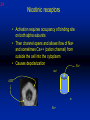
1 Cholinergic Receptors • Nicotinic – Skeletal muscle – Ganglia • Muscarinic Compliments of Byron Yoburn, Ph.D. 2 Cholinergic Receptors • Nicotinic: – Ligand-gated ion channel – A pentameric structure , , , – need to occupy 2 units (binding site) – Can distinguish the nAchR in ganglia and skeletal muscle 3 Cholinergic Receptors • Muscarinic: G-protein coupled receptor Inhibit Adenylyl Cyclase *(Gi) Stimulate Phospholipase C *(Gq) Regulate K+ channels *(Gi) 4 Cholinergic Receptors •Muscarinic: G-protein coupled receptor Several subtypes •Nicotinic: Ligand-gated ion channel Several subtypes From: Basic and Clinical Pharmacology 8th edition, B.G. Katzung; Lange 2001 5 Cholinergic Receptors Details From: Basic and Clinical Pharmacology 8th edition, B.G. Katzung; Lange 2001 6 Cholinergic Systems Don’t forget: The Neuromuscular Junction uses ACH and has a nicotinic Receptor on the Muscle 7 Martini, Fundamentals of Anatomy and Physiology, 5th Edition, Prentice Hall 2001 The Organization of the Sympathetic Nervous System 8 The Organization of the Sympathetic Nervous System Thoracic Lumbar Martinit, Fundamentals of Anatomy and Physiology, 5th Edition, Prentice Hall 2001 9 Sympathetic Synapses Note location of synapse 10 The Organization of the Sympathetic Nervous System: The Adrenal Medulla 11 The Adrenergic Synapse 12 The Adrenergic Synapse From: Basic and Clinical Pharmacology 8th edition, B.G. Katzung; Lange 2001 13 Adrenergic Receptors It will be important to understand how alpha and beta receptors From: Basic and and the subtypes are distinguished: Clinical Pharmacology •Originally by relative potency 8th edition, B.G. Katzung; Lange 2001 •Now by cloning 14 Pharmacologic Demonstration of Adrenoreceptor Types • The existence of alpha and beta receptors was originally proposed by Ahlquist in 1948 • Latter Lands et al 1967, suggested the beta1 beta2 distinction 15 Adrenergic Receptors 16 From: Basic and Clinical Pharmacology 8th edition, B.G. Katzung; Lange 2001 17 Miscellaneous Autonomic Issues • Pre versus postsynaptic receptors • Colocalization/Co-release of peptides and other transmitters vesicles in both cholinergic and adrenergic nerves contain other substances in addition to the primary transmitter (eg. VIP in cholinergic neurons within Enteric Nervous System– see Table 6-1 for others). in some cases, they provide a faster or slower action to supplement or modulate the effects of the primary transmitter these other substances can also participate in feedback inhibition of the same and nearby nerve terminals 18 Miscellaneous Autonomic Issues Dopamine receptors and the autonomic System – There are some DA receptors in cardiovascular system and the smooth muscle of the renal vasculature that can can improve renal blood flow – DA itself at higher doses will act at Beta1 receptors in the heart 19 Cholinergic Agonists Cholinoceptor activating Cholinesterase Inhibiting Chapter 7 20 Cholinergic Agonists Cholinoceptor activating *acetylcholine *bethanechol *carbachol cevimeline *pilocarpine Cholinesterase Inhibiting ambenonium demecarium donepezil *echothiophate edrophonium galantamine *neostigmine *physostigmine pyridostigmine rivastigmine tacrine 21 Cholinergic Systems • • • • Parasympathetic Nervous system Skeletal Muscle Autonomic Ganglia Some Sympathetic sites (few) • Classic Agonists – ACH – Nicotine (alkaloid) – Muscarine (alkaloid) 22 Cholinergic Receptors • Basically 2 types – Muscarinic: G-protein coupled – Nicotinic: Ligand gated ion channels • ACH is the agonist for both types • Originally distinguished based on sensitivity to alkaloid agonists • Were also distinguished using antagonists – Atropine a classic muscarinic antagonist – d-tubocurarine a classic nicotinic antagonist 23 Nicotinic Receptor subtypes • There are at least 2 subtypes – Ganglionic NN (Neuronal type) – Skeletal NM (muscle type) • Easiest way to distinguish is using selective antagonists – Hexamethonium = ganglionic blocker – Decamethonium = skeletal blocker • They are structurally similar, but can be separated using selective drugs 24 Nicotinic receptors • Activation requires occupancy of binding site on both alpha subunits • Then channel opens and allows flow of Na+ and sometimes Ca++ (cation channel) from outside the cell into the cytoplasm • Causes depolarization Na+ out ACH in Na+ 25 Muscarinic Receptor Subtypes • Appear to be at least 5 types • Are all G-protein coupled – M1 and M2 are sensitive to atropine, however the M1 receptor is selectively blocked by the antagonist pirenzapine (has use as a drug that reduces gastric acid secretion) – M1 appears to be found on nerves (CNS and some sympathetic postganglionic fibers (vasodilation??)) – M2 found in Heart and smooth muscle – M3 found in glands, vessels, smooth muscle – M4 and M5 are found in CNS?? And role is somewhat unclear 26 Muscarinic Receptors In general, seems to be 2 basic actions • ACH released from parasympathetic terminals will activate M receptors • ACH released from parasympathetic terminals can interact with presynaptic receptors on the terminals to reduce further transmitter release, – This presynaptic inhibition may be on parasympathetic terminals ( ACH) – OR on sympathetic terminals ( E / NE) 27 Intracellular Events Muscarinic Receptors • Various intracellular consequences of receptor activation – Activation of vascular M receptors which NO (EDRF) which activates Guanylyl cyclase to cGMP – Inhibition of cAMP formation (M2) – Activation of K+ channels (M2) – Activation of the Ca++-Phophoinositide signaling pathway (IP3-DAG pathway) (M1 and M3) • Receptor >G-protein>Phospholipase C>Converts PIP2 to IP3 and DAG Activates Release of Ca++ Activates PKC 28 Muscarinic Receptors Intracellular Messengers From: Basic Neurochemistry, 6th Edition, Siegel et al., eds., Raven Press 1999 29 Cholinergic Drugs From: Basic and Clinical Pharmacology 8th edition, B.G. Katzung; Lange 2001 30 Cholinomimetic Drugs • Can be direct or indirect Indirect: – primarily act via inhibiting the enzyme acetylcholinesterase which hydolyzes ACH to choline and acetate – degradation is rapid and obviously important, so this leads to enhancement of ACH action – There appear to be 2 types of inhibitors • Reversible: Competitive • Irreversible: include the “nerve gasses” and many insecticides, form covalent bonds with the enzyme 31 Cholinomimetic Drugs • Can be direct or indirect Direct: – bind to and activate receptors – in some cases can cause depolarization blockade (nicotinic) 32 Esters Directly Acting • 2 types – esters including ACH – alkaloids such as muscarine and nicotine • E.g., Pilocarpine, oxytremorine – Esters: • charged, poorly lipid soluble • poorly absorbed, generally resistant to hydrolysis • Have limited uses – improve gastric tone, stimulate urination, ophthalmic (Glaucoma) • Methacholine, carbamic acid, carbachol, bethanechol 33 Esters Directly Acting Vy rapidly hydrolyzed ; Large amounts must be infused intravenously to achieve Concentrations high enough to produce detectable effects Hydrolyzed less quickly than ACH but more quickly than Carbamic acid and its derivatives carbachol and bethanechol More resistant to hydrolysis by Acetylcholinesterase than ACH or methcholine The -methyl groups on methacholine and bethanechol reduces potency of the drugs at nicotinic receptors. 34 Directly Acting Esters 35 Cholinergic Agonists Directly acting • Alkaloids and Synthetics 36 Cholinergic Agonists Directly acting • Alkaloids and Synthetics – – – – typically well-absorbed have similar effects to other ACH agonists Differ in Muscarinic/Nicotinic specificity Uses are limited: • ophthalmic uses 37 Cholinergic Agonists Directly acting Pharmacodynamics: • Relatively predictable: – – – – – – – – eye CV system Respiratory GI Urinary CNS Neuromuscular Junction Glands Organ Response 38 Cholinergic Agonists Directly acting continued Pharmacodynamics: • Relatively predictable: – – – – – – – – eye CV system Respiratory GI Urinary CNS Neuromuscular Junction Glands Organ Response 39 Cholinergic Agonists Direct Some Pharmacological Properties of Choline Esters 40 Glaucoma Muscarinic agonists and AchE inhibitors reduce IOP by contracting the ciliary body and enhance fluid outflow, betaadrenergic blockers may also reduce secretion of aqueous humor by the ciliary epithelium • • Ciliary body (ciliary epithelium; Beta-adrenergic receptors Stimulate aqueous humor) The fluid leaves the anterior chamber at the angle where the cornea and iris meet When the fluid reaches the angle, it flows through a spongy meshwork and leaves the eye. Open-angle glaucoma= the angle that allows fluid to drain out of the anterior chamber is open. However the fluid passes too slowly through the meshwork drain. As the fluid builds up, the pressure inside the eye rises. Unless the pressure at the front of the eye is controlled, it can damage the optic nerve and cause vision loss. High pressure puts you at risk for glaucoma. It may not mean that you have the disease. http://www.nei.nih.gov/health/glaucoma/glaucoma_facts.htm 41 Cholinergic Agonists Indirectly acting • As a group they block ACH hydrolysis • Typically act at the enzyme, – but some compounds may also stimulate the N-Ach R • An Important group of drugs – mostly insecticides – some therapeutic application • There are pharmacokinetic differences and chemical structural differences • Pharmacodymanics are virtually identical 42 Cholinergic Agonists Indirectly acting Often classified as • Reversible: - Short acting edrophonium - Longer-acting pyridostigmine, neostigmine, physostigmine • Irreversible – The organophosphates: • Typically forms a covalent bond with the enzyme • The drug phosphorylates the enzyme – This phosphorous-enzyme bond is very stable and hydrolyzes very slowly • There appears to be a strengthening of the bond over time (“aging”) • Can be treated with pralidoxime which can reactivate ACHe if not AGED 43 Cholinergic Agonists Indirectly acting • Chemical groups: – quartenary ammonium alcohols – carbamic acid esters with quartenary or tertiary ammonium (e.g., neostigmine) – organic derivatives of phosphoric acid = the organophosphates 44 Cholinergic Agonists Indirectly acting • The therapeutic action of the oxime compounds (eg. pralidoxime) resides in their capacity to reactivate acetylcholinesterase without contributing markedly toxic actions of their own. Pralidoxime (2-PAM) O AChE R O P R P R R Pralidoxime (2-PAM) + AChE 45 Cholinergic Agonists Indirectly acting • If the R groups on the phosphorylated enzyme are modified by metabolism, the enzyme has “aged” and now that phosphorylated enzyme cannot be dephosphorylated!! Pralidoxime (2-PAM) O AChE O modifed P R P modified R Pralidoxime (2-PAM) + AChE 46 Cholinergic Agonists Indirectly acting •quartenary ammonium alcohols (e.g., edrophonium) •carbamic acid esters with quartenary or tertiary ammonium (e.g., neostigmine), •simple alcohols with a quartenary ammonium group (e.g., edrophonium) All reversible 47 Cholinergic Agonists Indirectly acting Organophosphorous Compounds Mostly irreversible 48 Cholinergic Agonists Indirectly acting Absorption issues – Most of the charged compounds are poorly absorbed (e.g., neostigmine – Thus these drugs are typically not a danger – The organophosphates are usually well-absorbed making them dangerous to humans. Absorbed by GI, lung, skin, membranes. echothiophate is an exception - rather poorly absorbed • The absorption properties make them effective insecticides • Some drugs (parathion, malathion) must be activated in vivo. (converted to oxygen analogs) – activated malathion is rapidly metabolized in mammals and birds, but not insects and fish. – Parathion is NOT deactivated 49 Cholinergic Agonists Indirectly acting Typical uses • Insecticides • glaucoma increased intraocular pressure which can damage the retina if untreated • mysthenia gravis disease which affects skeletal muscle neuromuscular junctions decreased number of functional nicotinic receptors in the neuromuscular junction. (autoimmune?) 50 Cholinergic Agonists Indirectly acting Organophosphorous compounds including Insecticides – DFP=isoflurophate very potent irrevers inhib – Tabun; extremely toxic nerve gas – Sarin extremely toxic nerve gas – Soman extremely toxic nerve gas – Paraoxon; active metabolite of parathion – Malaoxon; acitive metabolite of malthion – Parathion; insecticide to be phased out 10/03 – Diazinon=dimpylate; insecticide for gardening banned for indoor use, to be phased out 10/05 – Chlopyrifos; insecticide used in consumer products and limited non residential settings – Malathion; insecticide, generally safer than other agents – Echothiophate; extremely potent choline derivative; used in glaucoma 51 Insecticide Poisoning – You should know the signs of poisoning: – MUSCARINIC EXCESS: miosis, salivation, sweating, bronchial secretion and constriction, vomiting, diarrhea, – CNS effects follow: activation, convulsions, coma, respiratory arrest – Peripheral effects on skeletal N receptors are of especial concern: depolarizing blockade – IN short you die because: • obstruction of airway by secretions, Bronchospasm and laryngospasm • Neuromuscular blockade • CNS respiratory failure – Chronic exposure to organophosphates can cause neurotoxicity which includes sensory disturbances, ataxia, weakness, fatigue, – appears to be due to axonal degeneration and demylenation, these are not due to ACHe effects 52 Insecticide Poisoning • Treatment is typically atropine • or pralidoxime – A reactivator – Seems to “pick-off” the phosphorous group on ACHe and thus reactivate it – Best when given to “fresh” phosphorylated ACHe – Seems to work preferentially at the skeletal N receptor 53 Cholinergic Agonists Indirectly Acting