* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1 - Catalysis Eprints database
Physical organic chemistry wikipedia , lookup
Bottromycin wikipedia , lookup
George S. Hammond wikipedia , lookup
Metal carbonyl wikipedia , lookup
Discodermolide wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Petasis reaction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Ene reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Hydrogenation wikipedia , lookup
Stille reaction wikipedia , lookup
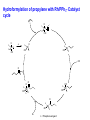
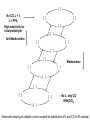
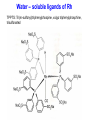
Homogeneous Catalysis HMC-4- 2010 Dr. K.R.Krishnamurthy National Centre for Catalysis Research Indian Institute of Technology, Madras Chennai-600036 Homogeneous Catalysis- 4 Hydroformylation Oxo reaction Reaction of CO and H2 with terminal alkene Production of n-butyraldehyde, butanols, C6- C9 alcohols & Long-chain fatty alcohols Hydroformylation- The beginning Journey from Fischer- Tropsch to Hydroformylation / Oxo reaction Discovery of Hydroformylation Hydroformylation- Discovery Hydroformylation-History 1938 Otto Roelen found the Hydroformylation At first cobalt-catalyst 1965 Wilkinson found rhodium catalyst 1980 process had been upgraded RCH/RP-process- separation of rhodium from the product Also named oxo-process Useful to converting terminal alkenes into other organic products Carbon chain increased by one Main industrial application is in the production of butanal from propene Hydroformylation-Applications Oxo process- For plasticizer alcohols Hydroformylation Addition of a H atom and a formyl group to the double bond of an alkene. Efficient with terminal alkene R → + CO + H2 R CHO + R CHO “normal” “iso” linear product branched product Thermodynamics: Hydrogenation Vs. Hydroformylation H2 + CH3CH=CH2 ∆G ∆H → 63 21 CH3CH2CH3 -25 -105 H2 + CH3CH=CH2 + CO → ∆G ∆H 63 21 -138 -109 = -88 kJ mol-1 = -126 kJ mol-1 CH3CH2CH2CHO -117(1) = -42 kJ mol-1 -238 = -150 kJ mol-1 Though alkane is the thermodynamically more favourable, the actual product is the aldehyde, because “kinetic” control occurs. The reaction is highly exothermic and is conducted at adiabatic conditions. 70 years of Oxo synthesis Five quantum leaps of development: 1. “Diaden process” with heterogeneous cobalt catalysts; 2. High pressure process with homogeneous Cobalt catalyst; 3. Introduction of Rh as the central atom of complex catalysts; 4. The ligand modified Rh or Co catalysts; and 5. The two-phase catalysis Plasticizer alcohols via Hydroformylation Hydroformylation - Features Preparation of Co complex Hydroformylation of propene with Cobalt catalyst HCo(CO)3 ( ) -CO HCo(CO)4 Co(CO)3(nPr) HCo(CO)3 Co(CO)3(iPr) nPrCHO Co(CO)3(COPrn) CO H2 Co(CO)3(COPri) iPrCHO H2 is propylene The inner cycle shows the formation of liner product and the outer cycle the Branched isomer Cobalt catalyzed hydroformylation- Mechanism Co2(CO)8 + H2 ⇋ (high pressure) 2[CoH(CO)4] 1. The complex loses CO to produce the coordinatively unsaturated complex catalyst. 2. The complex catalyst coordinates to the alkene (Propene). 3. Undergoes insertion reaction → n-alkyl complex (may be branched also). 4. Propene coordination followed by olefin insertion into metal-H bond in a Markovnikov or anti-Markovnikov fashion gives the branched or the linear metal-alkyl complex, respectively. 5 At high pressures of CO, the complex undergoes migratory insertion of CO to yield the acyl complex (IR spectral evidence). 6. Attack by H2, as strongly acidic HCo(CO)4 will yield the aldehyde and regenerate. 7. The last step is often rate-determining. Cobalt catalyzed hydroformylation- Mechanism The catalytic cycle is firmly established. Both Co(CO)4 (COPrn) and Co(CO)4 (COPriso) have been isolated and their reactions with H2 as well as HCo(CO)4 have been studied. There are spectroscopic data and/or strong theoretical arguments in favour of the existence of all the catalytic intermediates. Hydroformylation- General features There are two ligands, CO and L = PPh3. Hydroformylation with Rh can also be effected in the absence of phosphine. In such a situation, CO acts as the main Ligand. The intermediate complexes in the catalytic cycle switch from 18e- to 16 ecomplexes The catalyst precursor undergoes ligand disssociation to generate coordinatively unsaturated species. There are two insertion steps (alkene and CO) The selectivity to n-butyraldehyde is determined in the first insertion step. This is then followed by oxidative addition step (of H2), which is the ratedetermining step. Reductive elimination gives the aldehyde and generates the catalyst General catalyst composition- Hx My (CO)n Ln Hydroformylation activity pattern with unmodified ligand (n=0) Rh >> Co >Ir,Ru > Os >Pt >Pd > Fe >Ni Rh & Co most commonly used; Pt asymmetric hydroformylation Reaction cycle for hydroformylation on Co Mechanism with long chain olefins, > C3 Mechanism on Rh catalysts Approach of Propylene leads to isomers Ligand effects Steric effects by ligands Ligand effects Steric (cone angle bite angle), substituents & electronic factors of ligands affect the activity & n/i ratio Product selectivity: Linear Vs. Branched aldehydes: Regio-selectivity Two different ways of inserting an alkene into a metal-hydrogen bond: H R + R → Rh → Rh Rh R AntiMarkovnikov Markovnikov It is considered to be primarily an effect of steric crowding around the metal center. The normal alkyl requires less space and therefore formed more easily than the branched one in the presence of bulky ligands. Higher selectivity for the linear aldehyde by the addition of alkylphosphine Replacement of CO by a bulky ligand disfavours the formation of complexes of sterically crowded 2-alkane Co(CO)2PBu3 CHCH2CH2CH3 isomerize→ Co(CO)2PBu3 CH3CH-CH2CH3 k << 1 Phosphine derivatives as ligands for Co catalysts Phosphine derivatives -PR3 – R = C6 H5, n-C4H9 Variations in Gr V elements Ph3P >> Ph3 N > Ph3 As > Ph3Sb> Ph3Bi; Phosphine ligands –Best choice Alkyl phosphines Strong electron donors and thus dissociation of CO is retarded, Leads to more stable catalysts, but also much slower catalysts. Aryl phosphines Do not seem to be very effective ligands. They are weaker electron donors and form less stable complexes in the competition with CO They quickly decompose at higher temperatures. The more electron withdrawing the aryl group is, the faster the decomposition. The reported order of activity (195oC, 36 bar) Ph2EtP > PhBu2P > Bu3P > Et3P > PhEt2P > Cy3P The linear .Vs. branched ratio decreases as follows: Bu3P > Et3P = PhEt2P = Cy3P = PhBu2P > Ph2EtP, ranging from 5.5 to 3 N containing ligands like amines, amides & isonitriles show lower reaction rates due to stronger bonding with metal The Rh Catalyst Rh phosphine complexes are more active catalysts than cobalt complexes → [RhH(CO)(PPh3)3 [RhH(CO)(PPh3)2] + PPh3 Lower temperatures and Pressures Higher selectivity to linear aldehydes Catalyst separation is made easier Rate expression: d[RCHO]/dt = k[Rh]a[alkene]b[H2] / [CO] Value of b depends on the alkene; both a and b may be less than one. Inverse dependence of rate on the concentration of CO is observed only at total pressure exceeding 40 atm. At lower pressures, the rate increases with increase in pressure. Industrially, a large excess of the phosphine is used, which is necessary for a good selectivity to n-butyraldehyde. Hydroformylation of propylene with Rh/PPh3- Catalyst cycle L L L L H -L H Rh CO H Rh OC L L Rh L CO L OC Rh L CO O H L OC H O L Rh L H OC O L OC H2 Rh L L = Phosphorous ligand CO Rh L Mechanistic insights In-situ IR and multinuclear NMR studies under less severe conditions: The oxidative addition of dihydrogen is most likely the rate-determining step Hydroformylation of 3,3,dimethyl1-butene 1-Octene CH3 C=C–C–C CH3 O have been identified by NMR Rh(COR)(CO)4 intermediate is seen by IR Styrene O Ph C C RhL2(CO)2 RhL2(CO)2 [ ] No CO, L = 3 L = PPh3 High selectivity for n-butyraldehyde [ ] [ ] [ ] [ ] Anti-Markovnikov [ ] [ ] [ ] [ ] Markovnikov [ ] [ ] [ ] [ ] [ ] [ ] [ ] ←No L, only CO HRh(CO)4 Schematic drawing of catalytic cycles coupled by substitution of L and CO in Rh catalyst In a typical industrial process, the Rh to phosphorus molar ratio is between1:50 to 1:100, while the partial pressure of CO is about 25 bar. All the complexes with the general formula, HRh(CO)nL4-n n = 0 – 4) might be present with variable concentrations. In fact, even HRh(CO)4 without any phosphorus ligand can be used as catalyst. The selectivity towards anti-markovnikov product increases with more phosphinated intermediates, whereas more carbonylation shifts the selectivity towards Markovnikov product. This is to be expected in view of the fact that sterically crowded environment around the metal center favours anti-Markovnikov addition and Yields n-aldehyde (linear aldehyde) Commercial homogeneous Oxo processes Cobalt catalyzed Hydroformylation is the old Workhorse The key issue in the hydrofomylation reaction is the ratio of the linear to the branched products (Regio-selectivity) Linearity can be influenced and maximized by influencing the kinetics and changing the ligand characteristics. Three major process types The first processes were based on cobalt carbonyl complexes. The second is the process based on phosphorus modified Co-based catalyst system developed by Shell. Third Rh based catalysts - Ruhrchemie/Rhone-Poulenc, MitsubishiKasei, Union Carbide and Celanese Supported liquid phase & supported aqueous phase catalysts Oxo processes – The evolution Process Parameters Cobalt Carbonyl Cobalt +Phosphine Rhodium +Phosphine Temperature,oC 140-180 160-200 90-110 Pressure, atm. 200-300 50-100 10-20 Alkane formation Low Considerable Low Main product Aldehyde Alcohol Aldehyde Selectivity to n-butyraldehyde 75-80 85-90 92-95 Difficult HCo(CO)4 is volatile Less difficult Isolation of catalyst Less difficult: Water soluble phosphine, a major advancement Oxo processes- Variations Aqueous biphasic catalysis: Ruhrchemie/Rhone-Poulenc Oxo process In aqueous, homogeneous two-phase catalysis, the active catalyst for the reaction is (and remains) dissolved in water. The reactants and the products, which are ideally organic and relatively non-polar, can be separated off after the reaction is complete by simply separating the second phase from the catalyst solution, thus making it easy to recirculate the latter. Positive influence of water and Inherent advantages of homogeneous catalysis. The new oxo process was completely different from the earlier ones : a) The catalyst is applied in water soluble form; b) The process procedure; c) The reactor with special mixing of the two-phases; d) The energy balance; and e) Simple circulation of the aqueous catalyst solution. For the water soluble ligands, the price and the performance are the key Water – soluble ligands of Rh TPPTS: Tri(m-sulfonyl)triphenylphospine, vulgo triphenylphosphine, trisulfonated Hydroformylation with TPPTS New generation oxo process- Features 1. High selectivity, producing virtually exclusive aldehydes with up to 96% linearity; 2. Simplicity in terms of apparatus and process operation; 3. Net steam supplier, making excellent utilization of the heat of the reaction by means of the “heat transfer medium” n-butyraldehyde; 4. Very simple recycling of the homogeneous oxo catalyst by immobilization using the “mobile support” water; 5. Excellent economics owing to minimal losses of the catalyst metal, Rh; 6. Low purity demands on the reactants; 7. Excellent process potential from safety and environmental points of view. Synthesis of TPPTS Sulfonation of triphenylphosphine: pH controlled selective extraction and Re-extraction procedures; Further Improvement in ligand synthesis BISBIS: sulfonated bis(diphenylphosphine)biphenyl, varying grades of sulfonation; BINAS: sulphonated NAPHOS, binaphthyl structures Rh/BINAS is the most active and selective water-soluble hydroformylation catalyst. Its reactivity is up to 10 times higher than TPPTS with n/iso selectivities of 98/2 compared to 96/4 with TPPTS Hydroformylation- Process variations The Shell Process Higher alkene feed → Detergent alcohol Phosphine-modified Co catalyst for production of linear alcohols with 12-15 C atoms Important characteristics: 1. HCo(CO)3(P n-Bu3) is less active for hydroformylation than HCo(CO)4, but more active for subsequent hydrogenation of the aldehyde. 2. Both hydroformylation and hydrogenation of the aldehyde are catalyzed by the same catalyst. 3. Phosphorus ligand substituted derivatives are more stable than their carbonyl analogues at high temperatures and lower pressures. Linear alcohols - Chemical considerations The internal alkenes must be isomerized to terminal one from the thermodynamic mixture, where the terminal alkene will be very small. The hydrofomylation catalyst must have a very strong preference for the terminal carbon atom to give 60-80% linear products. HCo(CO)4 is an effective isomerization catalyst. The terminal alkenes undergo faster or preferential formation of 1-alkyl group or a faster migration of the 1-alkyl group. The activity of 1-alkene is much higher than that of internal alkenes (1000 times faster). Linear Alcohols by Hydroformylation of internal alkenes Thermodynamic Vs. Kinetic Pull H2 + CO → Slow→ O Very fast ↑ ↓ HCo(CO)4 H2 + CO → Fast→ O Cobalt, once attached to an alkene, “runs” along the chain until an irreversible Insertion of CO occurs (Chain running). Alkene does not dissociate from the cobalt hydride during the isomerization process. Ligand substitution on the Co carbonyl by tert-alkyl phosphines: Shell process The reaction is a hundred times slower, the phosphine complex is less active; Hence higher temperatures, 170oC versus 140oC The selectivity to linear products increases (75-90% versus 60-70%) The carbonyl complex formed, HCoL(CO)3 is much more stable; Hence the process is operated at lower pressure (25-100 bar versus 200-300 bar) The catalyst acquires activity for hydrogenation Isomerization Other Hydroformylation reactions O AcO 'Rh' OAc H2, CO O OAc H H OAc 'Rh-L' H2, CO O Vitamin A OH H H2, CO OH HO 1, 4 Butane diol OH 'Rh-L' OH OAc O OH OH 3-methyln1, 5 pentane diol 1. BASF/Hoffmann-LaRoche;Intermediate for Vitamin A;Rh catalyst without Phosphorus 2. ARCO; An inermeidate for 1,4-butanediol; Rh with Phosphorus ligand 3. Kuraray; An intermediate for 3-Me1,5-pentanediol; Rh with CO and Phosphorus 4. Mitshubishi Kasei; Isononyl aldehyde; Rh catalyst with PPh3 oxide as a weak ligand Homogeneous catalysts