* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry II Introduction
Discodermolide wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Elias James Corey wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
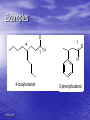
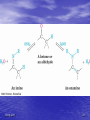
Organic Chemistry II Aldehydes and Ketones Dr. Ralph C. Gatrone Department of Chemistry and Physics Virginia State University Spring, 2011 1 Chapter Objectives • Nomenclature • Preparation • Reactions • Spectroscopy Spring, 2011 2 Nomenclature • Aldehydes • Identify the alkane • Parent alkane must contain the CHO group • CHO group C is numbered 1 • Replace the “e” with “al” Spring, 2011 3 Examples O 1 4 1 H O H 4-butyloctanal Spring, 2011 3-phenylbutanal 4 Nomenclature • Aldehydes • Aldehyde carbon is bonded to ring • Suffix used is “carbaldehyde” Spring, 2011 5 Examples O O H benzenecarbaldehyde Spring, 2011 H cis-2-methylcyclopentanecarbaldehyde 6 Common Names O H H H formaldehyde valeraldehyde O O H3C H acetaldehyde O H acrolein H crotonaldehyde O CH3CH2 H propionaldehyde O O butyraldehyde O H H O H benzaldehyde Spring, 2011 cinnamaldehyde 7 Nomenclature • • • • • • Ketones Identify the alkane Parent alkane The longest chain containing the carbonyl group The carbonyl C gets the lowest number possible Replace the “e” with “one” Spring, 2011 8 Examples O 3-heptanone O (E,E)-nona-5,7-dien-2-one O O O 1,2-cyclohexandione Spring, 2011 2-cyclohexenone 9 Nomenclature • If present with another functional group • Prefix “oxo” is used O O 4-oxohexanal Spring, 2011 10 Common Names O O O acetone Spring, 2011 acetophenone benzophenone 11 As a Substituent • When R-C=O is used as a substituent • Referred to as an acyl group • Ending “yl” is used O O acetyl Spring, 2011 benzoyl 12 Preparation • [O] of primary ROH PCC/CH2Cl2 OH O • [H] of RCO2H o OH 1. DIBAH/toluene/-78 C O 2. H3O+ O • [O] of secondary ROH OH [O] O many reagents can be use cost, scale, sensitivity to acid or base Spring, 2011 13 Preparation • Ozonolysis of Alkenes R H 1. O3 2. Zn/HOAc H R O must have one H Spring, 2011 14 Hydration of Alkynes • Hydration of terminal alkynes in the presence of Hg2+ (catalyst) Spring, 2011 15 Preparation From Organometallics O R O R2CuLi R Cl R R2CuLi from RLi and CuCl O R O R2Cd Cl R R R2Cd from RLi and CdCl2 Spring, 2011 16 Preparation • Friedal-Crafts Acylation O R O Cl R AlCl3 • Recall: • Reaction does not occur on deactivated rings Spring, 2011 17 Reactions • Oxidation of Aldehydes O R O [O] H R OH • [O] = KMnO4/acid; hot HNO3, and CrO3/acid • Ketones are generally inert to oxidation Spring, 2011 18 Reactions O O - + • Resonance contribution • Carbon is electrophilic • Oxygen is nucleophilic Spring, 2011 19 Nucleophilic Addition O O - Nu Nu: • Provides a tetrahedral intermediate Spring, 2011 20 Spring, 2011 21 The Tetrahedral Intermediate HA R R O R R OH Nu Nu HA R R OH Nu HA H + OH R R -H2O Nu Nu R R • Aldehydes are more reactive than ketones • Consider several nucleophiles Spring, 2011 22 Nucleophile = Water • • • • Product is a 1,1-diol, a gem-diol, a hydrate Reaction is equilibrium process Position of equilibrium depends upon structure Reaction is readily reversible O R Spring, 2011 R HO OH R R 23 Equilibrium Process O R OH H2O R H H OH O R OH H2O R R R OH when R=R=H 99.9% hydrate when R = R = CH3 99.9% carbonyl Spring, 2011 24 Nucleophile = Y in HY • Reaction of C=O with H-Y, where Y is • electronegative, gives an addition product Formation is readily reversible Spring, 2011 25 Nu = HCN Cyanohydrin Formation • HCN – very weak acid – – – – pKa = 9.1 Equilibrium favors HCN Availability of CN as nucleophile is reduced Base catalysis favors cyanohydrin formation Spring, 2011 26 Uses of Cyanohydrins • The nitrile group (CN) can be reduced with LiAlH4 to yield a primary amine (RCH2NH2) • Can be hydrolyzed by hot acid to yield a carboxylic acid Spring, 2011 27 Nucleophile = Organometallic Reagent • Grignard reagent • Effectively a carbanion Spring, 2011 28 Grignard Additions O R MgX+ O H R R' MgX OH R H H3O+ R' H R' secondary alcohol O R O R" MgX OH R' R" R R" R R' MgX+ H3O+ R' tertiary alcohol Spring, 2011 29 Nucleophile = Hydride • Reduction of Carbonyl compounds • Can use NaBH4 or LiAlH4 Spring, 2011 30 Hydride Addition • • • • Convert C=O to CH-OH LiAlH4 and NaBH4 react as donors of hydride ion Source of H-1 (not real but useful formally) Protonation after addition yields the alcohol Spring, 2011 31 Nucleophile = Amine Imine and Enamine Formation • Amines – organic derivatives of ammonia • Classified by number of substituents on N .. N H H H ammonia .. N .. N R H H primary R .. N R H secondary R R R tertiary • Primary and Secondary amines react • Tertiary amines do not react with carbonyls Spring, 2011 32 Spring, 2011 33 Imines and Enamines • Requires an acid catalyst • pH dependent reaction • Reaction is slow at high and low pH • At high pH – not enough acid to protonate • At low pH – the amine is protonated Spring, 2011 34 Spring, 2011 35 Imine Formation is Reversible • Drive reaction to right – Add excess amine – Remove water • Dean Stark Trap – Removes water – Azeotrope formation Spring, 2011 36 Derivatives of Imines • Hydroxylamine (NH2OH) O R NOH NH2OH R' H+ R R' oxime • Hydrazine (NH2NH2) O R Spring, 2011 NH2NH2 R' H+ NNH2 R R' hydrazone 37 Uses of Oximes • Beckmann rearrangement • Synthesis of Nylon NHOH H+ H N O Nylon caprolactam Spring, 2011 38 Uses of Hydrazones The Wolff–Kishner Reaction • Reduction under basic conditions • Ketone or Aldehyde into an alkane • Originally carried out at high temperatures but with dimethyl sulfoxide as solvent takes place near room temperature Spring, 2011 39 Uses of Hydrazones The Clemmensen Reduction • Reduction under acidic conditions NNH2 R H Zn(Hg)/HCl H R • Provides alkane from Ketone/aldehyde • Through Hydrazone Spring, 2011 40 Uses of Hydrazones Reduction of Carbonyls • Reduction under neutral conditions – Tosylhydrazone NaBH3CN O O S NNH2 TsNHN O H3C Spring, 2011 41 Nucleophile = Alcohol • Two equivalents of ROH and acid catalyst • Acetal formation O OR ROH/H+ OR H3O+ HO O OH H+ O O H3O+ Spring, 2011 42 Uses of Acetals • Acetals can serve as protecting groups for aldehydes and ketones • It is convenient to use a diol, to form a cyclic acetal (the reaction goes even more readily) Spring, 2011 43 Uses of Acetals • Thioacetals • Prepared in same manner as acetals • Reduction under neutral conditions SH HS Raney Ni S O Spring, 2011 acid S 44 Acetals and Hemiacetals • Common in carbohydrate chemistry CH2OH O HO OH H H OH H OH CH2OH D-Fructose Spring, 2011 O OH HO OH HOCH2 O OH + HO CH2OH CH2OH OH PYRANOSE 72% FURANOSE 28% 45 Glucopyranoses OH OH O HO HO HO O OH OH HO OH OH H -D-Glucose Spring, 2011 H -D-Glucose 46 Glucose -D-glucopyranose • mp = 146 oC and [] = +112.2o -D-glucopyranose • mp = 148 - 155 oC and [] = +18.7o • Dissolve either in water, mutarotation occurs • Alpha become beta, beta becomes alpha • Equilibrium mixture results (37:63 :) Spring, 2011 47 Some Phosphorus Chemistry • Amines react with alkyl halides – Quaternary ammonium salt .. N R R RX R R R N + R X- R • Phosphines also react with alkyl halides R .. P R Spring, 2011 R RX R R P + R X- R 48 Phosphorus Chemistry • Positive charge on P stabilizes negative charge that can form on an alpha carbon (must have a H atom) R R P + base R X- CH2R R R P + R - CHR an ylid alpha carbon • Ylides are nucleophilic • React with carbonyl compounds Spring, 2011 49 Nucleophile = Phosphorus Ylide The Wittig Reaction O R R P + R - R R - CHR an ylid O + PR3 CHR R R betaine O R PR3 H R CHR R oxaphosphetane R CHR + R3P=O • Extends carbon chain by one carbon atom • Adds a double bond into system • Known to be able to control stereochemistry of double bond Spring, 2011 50 Nucleophile = Phosphorus Ylide The Wittig Reaction • Generally use triphenyl phosphine • Triphenylphosphine oxide is very stable • thermodynamically Formation of P=O releases energy Spring, 2011 51 Uses of the Wittig Reaction • Can be used for monosubstituted, disubstituted, and • trisubstituted alkenes but not tetrasubstituted alkenes For comparison, addition of CH3MgBr to cyclohexanone and dehydration with, yields a mixture of two alkenes Spring, 2011 52 Brief Review • Aldehydes and ketones react with Nu • sp3 intermediate forms • Intermediate may – Reverse to give SM – Accept proton to form addition product – Eliminate water to form new Nu=C species • If derivative of carboxylic acid, also may O R O Nu: X - R O X R Nu Nu • See details of this chemistry in Chapter 16 • Elimination of X does not occur when X = H or R Spring, 2011 53 Consider Leaving Groups • Hydroxide (HO-) is a poor leaving group – Generally forms water in order to leave • Hydride (H-) very poor leaving group – An exception found in Cannizzaro Reaction – Aldehydes with no alpha hydrogens react gamma H H alpha H H beta Spring, 2011 O 54 The Cannizzaro Reaction • Cannizzaro observed reaction in strong basic media • Td intermediate transfers hydride to another aldehyde • REDOX reaction Spring, 2011 55 The Biological Analogue of the Cannizzaro Reaction • Enzymes catalyze the reduction of aldehydes and • ketones using NADH as the source of the equivalent of HThe transfer resembles that in the Cannizzaro reaction but the carbonyl of the acceptor is polarized by an acid from the enzyme, lowering the energy barrier Enzymes are chiral and the reactions are stereospecific. The stereochemistry depends on the particular enzyme involved. Spring, 2011 56 Conjugate Nucleophilic Addition to ,Unsaturated Aldehydes and Ketones • A nucleophile can add to the C=C double bond of an ,-unsaturated aldehyde or ketone • conjugate addition, or 1,4 addition • The initial product is a resonancestabilized enolate ion, which is then protonated Spring, 2011 57 Conjugate Addition • Resonance explains conjugate addition O O - O - + + positive charge designates electrophilic site • Certain Nu: will add in 1,2 manner • Other Nu: add 1,4 manner Spring, 2011 58 Conjugate Addition of Amines • Primary and secondary amines add 1.4 to , unsaturated aldehydes and ketones to yield amino aldehydes and ketones Spring, 2011 59 Conjugate Addition of Alkyl Groups: Organocopper Reactions • Reaction of an , -unsaturated ketone with a lithium • • diorganocopper reagent gives 1,4 addition Diorganocopper (Gilman) reagents from by reaction of 1 equivalent of cuprous iodide and 2 equivalents of organolithium 1, 2, 3 alkyl, aryl and alkenyl groups react but not alkynyl groups Spring, 2011 60 Other Additions • Grignards add 1,2 and 1,4 to , - unsaturated ketones • Organo lithium reagents add 1,2 to , unsaturated ketones • Cyanide ion adds 1,4 to , -unsaturated ketones Spring, 2011 61 Summary of Reactions • Aldehydes - from oxidative cleavage of alkenes, oxidation of 1° • • • • • • • • • alcohols, or partial reduction of esters Ketones - from oxidative cleavage of alkenes, oxidation of 2° alcohols, or by addition of diorganocopper or organocadmium reagents to acid halides . Aldehydes and ketones - reduced to yield 1° and 2° alcohols , respectively React with Grignard reagents giving alcohols Addition of HCN yields cyanohydrins 1° amines add to form imines, and 2° amines yield enamines Reaction with hydrazine gives hydrazones – Reduction of hydrazone in base yields an alkane – Reduction of hydrazone in acid/Zn yields an alkane Alcohols add to yield acetals Phosphoranes add to aldehydes and ketones to give alkenes (the Wittig) -Unsaturated aldehydes and ketones are subject to conjugate addition (1,4 addition) Spring, 2011 62 Infrared Spectroscopy • C=O • Strong absorption 1660 – 1770cm-1 • See next two spectra Spring, 2011 63 Spring, 2011 64 NMR Spectroscopy • The aldehyde H resonates at 10d • Coupling is observed with adjacent H • J = 3Hz • H adjacent to C=O are slightly deshielded • Resonate near 2.0 to 2.3d • Methyl ketones are distinctive (2.1d) • See next spectrum Spring, 2011 65 Spring, 2011 66 NMR Spectroscopy • C=O carbons resonate between 190 – 215d • See next spectra Spring, 2011 67 Spring, 2011 68 Mass Spectrometry • A g H leads to McClafferty rearrangement • Positive charge remains with O fragment Spring, 2011 69 Mass Spectrometry cleavage is also observed • See next spectrum Spring, 2011 70 Spring, 2011 71