* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PPTB&W - Gmu - George Mason University
Lewis acid catalysis wikipedia , lookup
Chemical element wikipedia , lookup
Hydrogen bond wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Acid–base reaction wikipedia , lookup
Atomic orbital wikipedia , lookup
Water splitting wikipedia , lookup
Artificial photosynthesis wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Geochemistry wikipedia , lookup
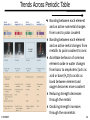
Coordination complex wikipedia , lookup
Bond valence method wikipedia , lookup
Biochemistry wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Oxidation state wikipedia , lookup
Electrolysis of water wikipedia , lookup
Bent's rule wikipedia , lookup
Electrochemistry wikipedia , lookup
Periodic table wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
History of chemistry wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Microbial metabolism wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Electronegativity wikipedia , lookup
History of molecular theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Halogen bond wikipedia , lookup
Electron configuration wikipedia , lookup
Extended periodic table wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Chemical bond wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Metalloprotein wikipedia , lookup
Atomic theory wikipedia , lookup
George Mason University General Chemistry 212 Chapter 14 Main Group Element Patterns Acknowledgements Course Text: Chemistry: the Molecular Nature of Matter and Change, 7th edition, 2011, McGraw-Hill Martin S. Silberberg & Patricia Amateis The Chemistry 211/212 General Chemistry courses taught at George Mason are intended for those students enrolled in a science /engineering oriented curricula, with particular emphasis on chemistry, biochemistry, and biology The material on these slides is taken primarily from the course text but the instructor has modified, condensed, or otherwise reorganized selected material. Additional material from other sources may also be included. Interpretation of course material to clarify concepts and solutions to problems is the sole responsibility of this instructor. 1/19/2015 1 Main-Group Elements Chapter Overview Application of bonding, structure, and reactivity to MainGroup Elements ● Hydrogen ● Period 2 Elements – Trends across Periodic Table ● Group 1A – The Alkali Metals ● Group 2A – The Alkaline Earth Elements ● Group 3A – The Boron Family ● Group 4A – The Carbon Family ● Group 5A – The Nitrogen Family ● Group 6A – The Oxygen Family ● Group 7A – The Halogens ● Group 8A – The Noble Gases 1/19/2015 2 Main-Group Elements In chemistry and atomic physics the periodic table divides the elements into 4 groups Main Group Elements ● s-block: 1 (IA); 2 (IIA) ● p-block: 13 (IIIA); 14 (IVA); 15 (VA) 16 (VIA); 17 (VIIA); 18 (VIIIA) Transition (d-block) Elements 3 4 5 6 7 8 9 10 11 12 (IIIB IVB VB VIB VIIB VIIIB VIIIB VIIIB IB IIB) Lanthanides (f-block Elements) ● Elements in Period 6, group 3 (IIIB) whose f-subshells are being filled Actinides (f-block Elements) ● Elements in Period 7, group 3 (IIIB) whose f-subshellls are being filled) 1/19/2015 3 Main Group Elements Group→ 1 ↓ Period IA 2 IIA 3 IIIB 1 4 IVB 5 VB 6 VIB 7 8 9 10 11 VIIB (VIIIB VIIIB VIIIB) IB Main Group Elements 12 2B 13 IIIA 14 IVA 15 VA 16 VIA 17 VIIA 18 VIIIA p block 2 d-block (Transition Metals) 3 4 5 6 7 s block f-block - Lanthanoid (ide) series) f-block - Actinoid (ide) series) 1/19/2015 4 Main Group Elements Periodic Table Numbering System Old Systems ● Old IUPAC (Used in Europe) Used Roman Numerals I, II, III, IV, V, VI, VII, VIII) and Letters (A &B) to indicate group (columns) The numbers roughly indicated the highest oxidation state of the elements, thus similar chemical properties The letters A and B were designated to the left (A) and right (B) part of the table ● CAS System (Used America) Similar to Old IUPAC except that the letter “A” referred to the Main group Elements and the letter “B” referred to the Transition Elements 1/19/2015 5 Main Group Elemetnts Periodic Table Numbering System Old systems confusing ● The use of the letters A & B in the old systems led to a lot of confusion New IUPAC System (Universally used) ● Numbers the groups increasingly from 1 -18 left to right on the standard periodic table incorporating the 10 Transition Elements groups ● These group numbers correspond to the number of s, p, and d orbital electrons added since the last noble gas element (in column 18) 1/19/2015 6 Main Group Elements Main Group Elements Elements that belong to the "s" and "p" blocks Counting the columns (groups 1- 8) across the table (ignoring the transition elements) gives 8 element groups which match the filling of the eight spaces for electrons in the ns and np subshells, ns2np6 One good aspect about the 1 to 8 group numbering system is that the group number indicates the number of valence (outer) electrons for atoms in the main group elements 1/19/2015 7 Main Groupd Elemtns The lightest Main Group members are represented by Helium, Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, And Fluorine Main group elements (with some of the lighter transition metals) are the most abundant elements on the earth, in the solar system, and in the universe They are sometimes called the representative elements 1/19/2015 8 Main-Group Elements Hydrogen (1s1) 90% of all atoms in Universe are Hydrogen atoms Single Electron; Small Size No perfectly suitable position in the periodic table Depending on the property, Hydrogen fits better in 1A, 4A, 7A 1/19/2015 +1 oxidation State (grp 1A? Relatively High Ionization Potential (grp 7?) Forms diatomic molecule (H2 - grp 7?) Shares electrons (grp 4?) Half-filled valence shell; ionization energy; electron affinity, electronegativity, and bond energies most similar to group 4 9 Hydrogen Chemistry Hydrogen Bonding Dipole-Dipole force between Hydrogen (H) and small, highly electronegative atoms with lone electron pair: Nitrogen (N); Oxygen (O); Fluorine (F) Highly reactive, combining with nearly every element Ionic (salt like) hydrides Group 1A & 2A metals 2Li(s) + H2(g) 2LiH(s) Lithium Hydride Ca(s) + H2(g) CaH2(s) Calcium Hydride In H2O, H- is a strong base that pulls H+ from water Na+H-(s) + H2O Na+(aq) + OH-(aq) + H2(g) Hydride ion is also a strong reducing agent Ti4+Cl4(l) + 4LiH(s) = Tio(s) + 4LiCl(s) + 2H2(g) 1/19/2015 10 Covalent Hydrides Hydrogen reacts with nonmetals to form covalent hydrides CH4 NH3 H2O HF Conditions for forming Covalent Hydrides depend on the reactivity of the nonmetal – the more stable, the more temperature & pressure required for formation Ex: Ammonia – 400oC & 250 atm Catalyst N2(g) + 3H2(g) 2NH3(g) Horxn = -91.8 kJ At low temperatures (-196oC) Hydrogen combines readily with reactive Fluorine (F2) F2(g) + H2(g) 2HF 1/19/2015 Horxn = -546 kJ 11 Metallic (Interstitial) Hydrides Many Transition Elements form metallic (interstitial) hydrides, where Hydrogen molecules (H2) and Hydrogen atoms (H) occupy the holes in the metal’s crystal structure. These are not compounds, but rather gas-solid solutions They lack a Stoichiometric formula because metal can incorporate a variable amount of hydrogen, depending upon temperature and pressure 1/19/2015 12 Trends Across Periodic Table Electrons fill 1 ns – 3 np orbitals according to Pauli Exclusion Principle and Hund’s Rule d orbitals in lower Periods can be used to accommodate additional oxidation states Atomic size generally decreases 1st ionization potential increases Electronegativity increases Metallic character decreases with increasing nuclear charge Reactivity highest at right & left sides, less in middle Bonding - metallic covalent none (noble gas) 1/19/2015 Continued on next Slide 13 Trends Across Periodic Table 1/19/2015 Bonding between each element and an active nonmetal changes from ionic to polar covalent Bonding between each element and an active metal changes from metallic to polar covalent to ionic Acid-Base behavior of common element oxide in water changes from basic to amphoteric (acts as acid or base (H2O) to acidic as bond between element and oxygen becomes more covalent Reducing strength decreases through the metals Oxidizing strength increases through the nonmetals 14 Group 1A - Alkali Metals (ns1) Lithium (Li), Sodium (Na); Potassium (K); Rubidium (Rb); Cesium (Cs); Francium (Fr) Single electron relatively far from nucleus weak metallic bonding - attraction between delocalized electrons and metal-ion cores in crystalline structure Low melting points, soft consistency Reactive Metals Powerful reducing agents – lose 1 electron becoming 1+ cations, donating the electron to other elements ns1 configuration forms salts readily (+1 cations) Low Heat of Atomization (oHatom ) – Recall Lattice Energy) Energy to convert solid into individual gaseous atoms oHatom (Li>Na>K>Rb>Cs) 1/19/2015 15 Group 1A - Alkali Metals (ns1) Low Ionization Energy (IE) – Each alkali element has the largest size and the lowest IE in its Period Size of atom decreases considerably when valence electron is lost Lattice Energy – The atomic radius increases as you move down a group. Since the square of the distance is inversely proportional to the force of attraction, lattice energy decreases as the atomic radius increases For a given anion, the Lattice Energy become smaller as the cation becomes larger 1/19/2015 16 Group 1A - Alkali Metals (ns1) Solubility – Despite strong ionic attractions, the Group 1A salts are water soluble – attraction between the ions and the polar Water molecule creates highly Exothermic Heat of Hydration (Hhydr) Entropy – Entropy increases as ions disperse going into solution overcoming the high lattice energy Magnitude of Hydration Energy decreases as ionic size increases H = -Hhydr (Li+ > Na+ > K+ > Rb+ > Cs+ 1/19/2015 17 Group 1A - Alkali Metals (ns1) Anomalous Behavior of Lithium Lithium ion (Li+) is small and highly positive Dissociation of Lithium salts, such as LiF, Li2CO3, LiOH, and Li3PO4, in water is much more difficult than similar salts of sodium (Na) and Potassium (K) Only member of Alkali group that forms simple Oxide and Nitride, Li2O & Li3N, on reaction with O2 & N2 in air Only Lithium forms organo-metalic molecular compounds with hydrocarbon groups from organic Halides 2Li(s) + CH3CH2Cl(g) CH3CH2Li(s) + LiCl(s) 1/19/2015 18 Group 1A - Alkali Metals (ns1) Reactions & Compounds of Alkali Metals Alkali metals reduce Hydrogen in Water to form Hydrogen gas 2E(s) + 2H2O 2E+ + 2OH-(aq) + H2(g) Where E = any alkali metal (Li, Na, K, Rb, Cs) Reaction becomes more vigorous down group Alkali metals reduce oxygen, but product depends on the metal 4Li(s) + O2(g) 2Li2O(s) oxide K(s) + O2(g) KO2(s) superoxide Alkali metals reduce Hydrogen to form ionic hydrides 2E(s) + H2(g) 2EH(s) 1/19/2015 19 Group 1A - Alkali Metals (ns1) Reactions & Compounds of Alkali Metals Alkali metals (E) reduce Halogens (X) to form Halides 2E(s) + X2 2EX(s) X = F, Cl, Br, I) Sodium Metal (Na) can be produced from Molten NaCL and electricity 2NaCl(l) 2Na(l) + Cl2(g) Sodium Hydroxide (Lye) can be produced from Salt (NaCl), water (H2O) and electrolysis 2NaCl(s) + H2O(l) 2NaOH(aq) + H2(g) + Cl2(g) In an ion-exchange process, water can be “softened” by removal of dissolved hard-water cations to displace Na+ ions from a “resin” M2+(aq) + Na2Z(s) MZ(s) + 2Na+(aq) (M = Mg, Ca: Z = resin) 1/19/2015 20 Group 1A - Alkali Metals (ns1) atomic properties physical properties 1/19/2015 21 Group 2A - Alkaline Earth Metals (ns2) Be, Mg, Ca, Sr, Ba, Ra (E2+ ions) Oxides (except Be) give basic (alkaline) solutions: Ca(OH)2, Mg(OH)2 High melting points (higher lattice energy than 1A) Atomic & Ionic sizes Smaller radii and higher ionization energy Increase in size down the group Combination of size, extra electron, and metallic bonding result in stronger attractions between delocalized electrons and the atom cores Thus, Melting Points and Boiling Points are much higher than 1A alkali metals Harder & more dense than Alkali metals, but soft and lightweight compared to transition metals (Fe, Cr, etc) 1/19/2015 22 Group 2A - Alkaline Earth Metals (ns2) Even though the Alkaline Earth metals have higher ionization potential, they still form ionic compounds (E2+), but Beryllium (Be) is an exception forming covalent bonds Like Alkali metals, Alkaline Earth metals are strong reducing agents Group 2A (Alkaline Earth) elements are reactive because the higher lattice energy of their compounds more than compensates for the large total Ionization Energy (IE) needed to form the 2+ cations The higher Lattice Energy (from the smaller cation size) and higher Charge Density results in lower solubility Ion-Dipole attraction is so strong that many slightly soluble 2A salts crystallize as “Hydrates” Epsom salt – MgSO47H2O Gypsum – CaSO42H2O 1/19/2015 23 Group 2A - Alkaline Earth Metals (ns2) The anomalous behavior of Beryllium Beryllium has smallest size; highest Ionization energy, and highest Electronegativity of the Alkaline Earth elements Combined with the high charge density of the ion (Be2+) it polarizes the nearby electron clouds very strongly and causes extensive orbital overlap; this results in covalent bonding BeF2 is the most ionic of the Beryllium compounds, but its melting point and electrical conductivity are relatively low compared to the other alkaline earth Fluorides Unlike the other Alkaline Earth Metals, whose oxides are basic, BeO is amphoteric and does not react with water to form OH- ions 1/19/2015 24 Group 2A - Alkaline Earth Metals (ns2) Diagonal relationships: Lithium and Magnesium Certain Period 2 elements exhibit behaviors that are very similar to those of the Period 3 elements immediately below and to the right 3 relationships 1. Li, Mg 2. Be, Al 3. B, Si Lithium and Magnesium reflect similar atomic and ionic size Both elements form: Nitrides, Hydroxides and Carbonates (CO3) that decompose with heat, Organic compounds with polar covalent metal-carbon bonds Salts with similar solubilities 1/19/2015 25 Group 2A - Alkaline Earth Metals (ns2) Reactions & Compounds (E = Mg, Ca, Sr, Ba) Metals reduce Oxygen (O2) to form Oxides 2E(s) + O2(g) 2EO(s) Ba + O2 BaO2 (Barium Peroxide) Larger metals reduce water to form hydrogen gas E(s) + 2H2O(l) E2+aq) + 2OH- (aq) + H2(g) Metals reduce Halogens to form ionic halides E(s) + X2 EX2(s) (X = F, Cl, Br, I) Most metals (Be exception) reduce Hydrogen to form ionic hydrides E(s) + H2(g) EH2 (s) 1/19/2015 (except Be) 26 Group 2A - Alkaline Earth Metals (ns2) Reactions & Compounds (E = Ca, Mg, Sr, Ba) Most elements reduce Nitrogen to form ionic Nitrides 3E(s) + N2(g) E3N2(s) (except Be) Element Oxides are Basic (except for amphoteric BeO) EO(s) + H2O(l) E2+(aq) + 2OH-(aq) All Carbonates undergo thermal decomposition to the oxide heat ECO3(s) EO(s) + CO2(g) (CaO – Lime) Beryl (Be3Al2Si6O18) - Gemstone, source of Be Magnesium oxide (MgO) – Refractory material for furnace bricks Alkyl Magnesium Halides – RMgX (R=Hydrocarbon) Grignard Reagents – organic compound synthesis 1/19/2015 27 Group 2A - Alkaline Earth Metals (ns2) atomic properties physical properties 1/19/2015 28 Group 3A – Boron Family (ns2np1) B Al Ga In Tl Boron heads family, but other elements in group 3A exhibit diverse properties Boron & Aluminum, especially Aluminum, are much more abundant than the others, but still quite rare Group 3A elements include “p” orbitals for first time In Period 4 (transition elements) the “d” orbitals are present Physical Properties are influenced by type of bonding 1/19/2015 29 Group 3A – Boron Family (ns2np1) Boron is a network covalent metalloid - Black, hard, very high melting point A network solid or covalent network solid is a chemical compound in which the atoms are bonded by covalent bonds in a continuous network In a network solid there are no individual molecules and the entire crystal may be considered a macromolecule Boron (metalloid) is much less reactive than the others members of the 3A group because it forms covalent bonds 1/19/2015 30 Group 3A – Boron Family (ns2np1) Other group members are metals – shiny, relatively soft with low melting points Aluminum is more ionic; its low density and 3 valence electrons make it a good electrical conductor Although Aluminum is a metal, its halides exist in the gaseous state as covalent dimers - AL2Cl6 (contrast salts of group 1 & 2 metals) Aluminum Oxide, Al2O3, is amphoteric (can act as an acid or base) rather than basic like the Group 1A & 2A metals Although the other Group 3A elements are basically ionic they exhibit more Covalent character than similar 2A compounds. 3A cations are smaller with more charge density than 2A cations and they polarize an anion’s electron cloud more effectively 1/19/2015 31 Group 3A – Boron Family (ns2np1) Oxidation-Reduction (REDOX) behavior in Group 3A Presence of Multiple Oxidation States ● In Groups 3A – 6A many of the larger elements (down the group) exhibit an oxidation state “two lower” than the A-Group number ● This lower state occurs when the atoms lose their np electrons, not the ns electrons. ● The lower oxidation state is the result of lower bond energies ● Bond energies decrease as the size of the atom and the bond length increase for elements lower in the group 1/19/2015 32 Group 3A – Boron Family (ns2np1) Increasing prominence of the low oxidation state ● When a group exhibits more than one oxidation state, the lower state becomes more prominent going down the Group ● All members of the 3A group exhibit the +3 state, but the +1 state appears first with some compounds of Gallium (Period 4) ● The +1 state becomes the most important state of Thallium (Period 6) 1/19/2015 33 Group 3A – Boron Family (ns2np1) Relative Basicity of Group 3 oxides ● Recall: A1 oxides (ionic charge +1 and more metallic) are more basic than A2 oxides (ionic charge +2 and less metallic) ● In general, oxides with the element in a lower oxidation state (less positive) are more basic than oxides with the element in a higher oxidation state ● For Indium oxides in Group A3, In+12O acts more like a metal and is more basic than In+32O3 ● The lower charge density of In+1 does not polarize the O-2 ion as much as the In+3 ion ● Thus, in E2O compounds, the E-O bonding is more ionic than in E2O3 compounds, thus; the O-2 ion is more available to act as a base – donate electron pair or accept a proton 1/19/2015 34 Group 3A – Boron Family (ns2np1) Boron Chemistry Boron compounds are covalent (unique within group) Forms network covalent compounds or large molecules with metals, H, O, N Electron deficient; uses two approaches to complete octet ● Accepting a Bonding Pair from Electron-Rich atom BF3(g) + NH3(g) F3B-NH3(g) (BF3 acts as acts as Lewis acid in accepting the electron pair from the Nitrogen in NH3) B(OH)3 + H2O(l) B(OH)4-(aq) + H+(aq) (Acts as acid by accepting electron pair from H2O) Note: Water is acting as the base 1/19/2015 35 Group 3A – Boron Family (ns2np1) Boron Chemistry Two approaches to filling octet (con’t) ● Accepting electron pair from Electron-Rich atom (con’t) Boron-Nitrogen compounds are similar in structure to elemental Carbon and some of its organic compounds Size, Ionization Energy, Electronegativity of Carbon is between Boron & Nitrogen Ethane & Amine – Borane have the same number & electron configuration C – C 1/19/2015 B – N 36 Group 3A – Boron Family (ns2np1) Boron Chemistry Two approaches to filling octet (con’t) ● Forming Bridge Bonds with Electron-Poor Atoms Boron Hydrides - Boranes 2 types of B – H bonds Normal electron-pair bond o sp3 orbital of B overlaps 1s orbital of H in each of the four terminal B-H bonds 1/19/2015 37 Group 3A – Boron Family (ns2np1) Hydride Bridge Bond (3-center, 2 electron bond) o Each B – H – B grouping is held together by only two electrons o Two sp3 orbitals, one from each B, overlap an H 1s orbital between them o Two electrons move through this extended bonding orbital – one from one of the B atoms and the other form the H atom – and join the 2 B atoms via the H atom bridge 1/19/2015 38 Group 3A – Boron Family (ns2np1) atomic properties 1/19/2015 physical properties 39 Group 3A – Boron Family (ns2np1) Reactions & Compounds Elements react sluggishly, if at all, with water (H2O) 2 Ga(s) + 6H2O(hot) 2Ga3+(aq) 6OH-(aq) + 3H2(g) 2Tl(s) + 2H2O(steam) 2Tl+(aq) +2OH-(ag) + H2(g) Note different oxidation numbers for Ga3+ & Tl+ All members form oxides when heated in pure O2 4E(s) + 3O2(g) 2E2O3(s) (E = B, Al, Ga, In) 4Tl(s) + O2 2Tl2O3(s) Oxide acidity decreases down the group: B2O3 > Al2O3 > Ga2O3 > In2O3 > TlO2 (weakly acidic) (strongly basic) The +1 oxide (TlO2) is more basic than the +3 oxide 1/19/2015 40 Group 3A – Boron Family (ns2np1) Reactions & Compounds All members reduce Halogens 2E(s) + 3X2 2EX3 (E = B, Al, Ga, In) 2Tl(s) + X2 2TlX(s) Trihalides of AL, Ga, In are mostly ionic but exist as dimers in the gas phase Acid (H2SO4) treatment of Al2O3 produces Al2SO4, a colloid (coagulant) used in water purification Al2O3 + 3H2SO4 Al2SO4(s) + 3H2O(l) 1/19/2015 41 Group 4A – Carbon Family (ns2np2) The whole range of elemental behavior occurs within the 4A group Non metalic Carbon (C) Metalloids (Silicon (Si) & Germanium (Ge) Metallic (Tin (Sn) & Lead (Pb) Newly synthesized element at bottom of group Carbon forms the basis of “Organic Chemistry” 20,000,000 compounds Polymer Chemistry Biochemistry based on Carbon Geochemistry Electronic technologies bases on Si 1/19/2015 42 Group 4A – Carbon Family (ns2np2) Bonding effects on Physical Properties Silicon has a much lower melting point than Carbon because of the longer, weaker bonds. The melting point difference between Germanium (Ge) and Tin (sn) is due to the change from network covalent to metallic Going from Group 3 to group 4 there are large increases in melting point and the Hfus because of the change from metallic to network covalent bonding 1/19/2015 43 Group 4A – Carbon Family (ns2np2) Allotropism: Different Forms of an Element Elemental Carbon – Graphite & Diamond Different crystalline & molecular forms with different physical properties ● Carbon Allotropes Graphite – Black, “greasy”, soft, more stable than diamond Diamond – Colorless, electrical insulator, extremely hard Bucky-Balls (Buckminsterfullerene) – soccer ballshaped with the formula C60 ● Tin Allotropes -tin – stable at room temperature & above -tin – stable below 13oC 1/19/2015 44 Group 4A – Carbon Family (ns2np2) Bonding Changes in Group 4A Carbon – Covalent (intermediate EN) Si & Ge – strong polar bonds (silicate minerals) Tin (Sb) & Lead(Pb) – Metallic (Ionic) Multiple Oxidation States Carbon (+4) Silicon (+4 more stable than +2) Lead (+2 more stable than +4) Elements with lower oxidation states act more like metals (more basic) 1/19/2015 45 Group 4A – Carbon Family (ns2np2) Highlights of Carbon Chemistry Carbon, like other elements in “Period 2, is the anomalous element in the group Carbon forms bonds with: ● Smaller Group 1A & 2A metals ● Many transition metals ● Halogens ● Neighbors B, Si, N, O, P, S ● Exhibits all possible oxidation states from +4 in CO2, and Halides to -4 in CH4 1/19/2015 46 Group 4A – Carbon Family (ns2np2) Highlights of Carbon Chemistry Two main features of Carbon Chemistry ● Catenation and the ability of Carbon to form multiple bonds Carbon can form chains, branches, and rings (aromatic & aliphatic) Multiple bonds – sigma (), Pi (), Triple () The C-C bond is short enough for side-to-side overlap of two half-filled 2p orbitals to form bonds that give rise to many diverse structures and reactivities of organic compounds 1/19/2015 47 Group 4A – Carbon Family (ns2np2) The Other 4A elements E-E bonds become longer going down the group, with decreasing bond strength C – C > Si – Si > Ge – Ge The empty d shell orbitals make these chains susceptible to chemical attack – they are reactive The long bonds are not suitable for overlap of p orbitals; thus, no bonds 1/19/2015 48 Group 4A – Carbon Family (ns2np2) Carbonates Metal Carbonates are the main mineral Marble, Limestone, Chalk, Coral, others Antacids – Calcium Carbonate & Stomach Acid CaCO3(s) + 2HCl(aq) CaCl2(aq) + CO2(g) + H2O(l) Limestone (CaCO3) deposits help moderate the effects of acid rain (H2SO4 & HNO3) Carbon Dioxide (CO2) Essential to all life as primary source of carbon in plants & animals through photosynthsis Atmospheric buildup from motor vehicles and fossil fuel powerplant severely affect global climate 1/19/2015 49 Group 4A – Carbon Family (ns2np2) Silicon Chemistry Silicon Halides are more reactive than Carbon Halides because Si (3s, 3p, 3d orbitals) has empty 3d orbitals available for bond formation The Si – X bond is long but stronger than corresponding C – X bond Si – X bond has some double bond character because of the presence of a bond and a different type of bond called a p,d- (side-to-side overlap of the Si d orbital and a Halogen p orbital Trimethylamine (CH3)3N 1/19/2015 trigonal pyramidal The impact of p,d- bonding on the structure of trisilylamine Trisilylamine (SiH3)3N trigonal planar 50 Group 4A – Carbon Family (ns2np2) Silicon chemistry is dominated by the Silicon-Oxygen – (Si–O) bond C – C bonds can repeat endlessly; similarly, the Si–O bonds can also repeat forming large chains in Silicate minerals in the earths crust and Silicones, which are synthetic polymers with a large number of industrial applications Silicate Minerals From common sand (SiO2) and clay to semiprecious amethyst, Silicate minerals are the dominant form of matter on the earth Oxygen is the most common element on earth and Silicon is the next most abundant 1/19/2015 51 Group 4A – Carbon Family (ns2np2) The Orthosilicate (–SiO4 –) grouping is the building unit for Silicate minerals Zircon ZrSiO4 1 Unit Hemimorphite 2 Units [Zn4(OH2Si2O7H2O] Beryl 6 Units [Be3Al2Si6O18] Silicon Polymers Manufactured Substances Alternating Si & O atoms with two Organic groups bonded to each Silicon atom 1/19/2015 52 Group 4A – Carbon Family (ns2np2) atomic properties physical properties 1/19/2015 53 Group 4A – Carbon Family (ns2np2) Important Reactions Group 4A elements are Oxidized by Halogens E(s) + 2X2 EX4 (E = C, Si, Ge) The +2 Halides are more stable for Tin (Sb) & Lead (Pb) SnX2 PbX2 The Elements are oxidized by Oxygen (O2) E(s) + O2(g) EO2 (E = C, Si, Ge, Sn) The oxides are more basic (metallic) going down the group Lead (Pb) forms the +2 oxide (PbO) - basic In natural streams, Carbon Dioxide (CO2) forms a weakly “acidic” solution CO2 + H2O ⇄ H2CO3(aq) ⇄ H+(aq) + HCO3-(aq) 1/19/2015 54 Group 4A – Carbon Family (ns2np2) Air & Steam passed over hot coke (carbon) produce gaseous fuel mixtures – producer gas & water gas C(s) + H2O(g) + air(g) CO(g) + CO2(g) + N2(g) + H2(g) Note: This industrial reaction cannot be balanced Hydrocarbons (C & H only) react with Oxygen (O2) to form CO2 & Water (H2O), a source of heat to yield steam (H2O) for electrical generation CH4(g) + 2O2(g) CO2(g) + 2H2O(g) + Heat Certain metal Carbides react with water to produce Acetylene (H-CC-H), used in oxyacetylene torches CaC2(s) + 2H2O(g) Ca(OH)2(aq) + C2H2(g) Acetylene is source material for organic compound synthesis and a fuel for Welding 1/19/2015 55 Group 4A – Carbon Family (ns2np2) Freon (chlorofluorocarbon) is formed from fluorinating Carbon Tetrachloride CCl4(l) + HF(g) CFCl3(g) + HCL(g) Production of Trichlorofluoromethane (Freon-11) is being discontinued because it is an atmospheric pollutant Silica (SiO2)is reduced to form elemental Silicon used in the manufacture of computer chips SiO2(s) + 2C(s) Si(s) + CO2(g) 1/19/2015 56 Group 5A – Nitrogen Family (ns2np3) Widest range of physical behavior in 1st 5 groups Compounds of Nitrogen (gaseous nonmetal) and Phosphorus (solid nonmetal) are important in industrial and environmental processes Arsenic (As) and Antimony (Sb) are network covalent metalloids with highest melting points in group Bismuth (Bi) exhibits metallic bonding Nitrogen (N) exists as diatomic molecules, which interact through very weak dispersion force producing a boiling point 200 oC below room temperature Phosphorus (P) is heavier and more polarizable than Nitrogen with stronger dispersion forces – higher melting point 44oC 1/19/2015 57 Group 5A – Nitrogen Family (ns2np3) atomic properties physical properties 1/19/2015 58 Group 5A – Nitrogen Family (ns2np3) Chemical Behavior in Group 5A Patterns Nitrogen forms a maximum of 4 covalent bonds The other elements in the group can expand the valence shell by using empty ‘d’ orbitals The noble gas configuration is attained by group 5 elements gaining 3 electrons – the first is Exothermic and the last two are Endothermic (requiring input of energy from surroundings As in groups 3A & 4A, fewer oxidation states occur moving down the group with the lower oxidation state becoming prominent Oxidation states ● Nitrogen – from +5 to -3 ● P, As, Sb – +5 & +3 ● Bi – +3 1/19/2015 59 Group 5A – Nitrogen Family (ns2np3) Nitrogen Chemistry Nitrogen Oxides – 6 stable forms 1/19/2015 60 Group 5A – Nitrogen Family (ns2np3) Nitrogen Oxoacids & Oxoanions Oxoacid Oxoanion 1/19/2015 61 Group 5A – Nitrogen Family (ns2np3) Nitric Acid (oxidizing agent) Reactions Active Metal (Al in dilute HNO3 solution) 8Al(s) + 30 HNO3(aq) 8Al(NO3)3 + 3NH4NO3 + 9H2O(l) Al(s) + 30H+(aq) + 3(N+5O3)-(aq) 8Al+3(aq) + 3(N+3H4)+(aq) + 9H2O(l) (net ionic equation) Less reactive metal (Cu), more conc HNO3 (N2+ forms) 3Cu(s) + 8HNO3(aq) 3Cu(NO3)2 + 4H2O(l) + 2N2+O(g) 3 Cu(s) + 8H+(aq) + 2(N+5O3)- 3Cu2+(aq) + 4H2O(l) + 2N+2O(g) (net ionic equation) Copper with still more concentrated HNO3 (N4+ forms) Cu(s) + 4HN+5O3(aq) Cu+2(NO3)2(aq) + 2H2O(l) + 2N4+O2(g) Cu(s) + 4H+(aq) + 2NO3-(aq) Cu+2(aq) + 2H2O(l) + 2NO2(g) 1/19/2015 (net ionic equation) 62 Group 5A – Nitrogen Family (ns2np3) Nitrates form when Nitric Acid (HNO3) reacts with the hydroxides, oxides, or carbonates of metals HNO3 + NaOH NaNO3 + H2O Nitrous Acid (HNO2), a much weaker acid, is formed from the reaction of a strong acid (HCl) and metal Nitrites NaNO2(aq) + HCl(aq) HNO2(aq) + NaCl(aq) Strong acid vs Weak acid – The more oxygen atoms bonded to the central nonmetal, the stronger the acid Oxygen atom pulls electron density from the Nitrogen atom, which in turn pulls electron density from the Oxygen of the O-H bond, facilitating the release of the H+ ion – The more Protons (H+) in solution, the stronger the acid. 1/19/2015 63 Group 5A – Nitrogen Family (ns2np3) Phosphorus Chemistry Phosphorus forms two important Oxides ● Tetraphosphorus Hexaoxide, P4O6 P (+3) has tetrahedral orientation with an Oxygen between pair of P atoms Reacts with water to form Phosphorus acid, H3PO3 P4(s) + 3O2(g) P4O6(s) + 6H2O(l) 4H3PO3(l) Only two of the H atoms are acidic, the third is bonded to the central P and does not dissociate Dissociation is complete in strong base solution 1/19/2015 64 Group 5A – Nitrogen Family (ns2np3) ● Tetraphosphorus Decaoxide (con’t) Phosphorus (+5) oxidation state P4(s) + excess 5O2 P4O10(s) P4O10(s) + 6H2O 4H3PO4(l) ● Phosphoric Acid (H3PO4) is a weak triprotic acid In water it loses one proton to form H2PO4 In excess strong base all three protons dissociate to form the Phosphate ion, PO43- + 3H+ 1/19/2015 65 Group 5A – Nitrogen Family (ns2np3) Diphosphate & Polyphosphates Polyphosphates are formed by heating Hydrogen Phosphates (ex. Na2HPO4) 2Na2HPO4(s) Na4P2O7(s) + H2O(g) The Diphosphate ion, P2O74-, is the smallest of the polyphosphates consisting of tetrahedral PO4 units linked through a common Oxygen 1/19/2015 66 Group 5A – Nitrogen Family (ns2np3) Reactions & Compounds Converting Nitrogen to other forms (fixing) is quite difficult because of the strength of the triple bond (NN) between the Nitrogen atoms. It can be fixed industrially by the Haber process N2(g) + 3H2(g) ⇄ 2NH3(g) Non Nitrogen Hydrides from metal Phosphides Ca3P2(s) + 6H2O(l) 2PH3(g) + 3Ca(OH)2 Halides formed by direct combination of elements 2E(s) + 3X2 2EX3 (E= P, As, Sb not N) EX3 + X2 EX5 (all except N & Bi with X = F & Cl) P4 in basic solution increases & decreases oxidation number P4(s) + 3OH-(aq) + 3H2O P3+H3(g) + 3H2P1+O2- 1/19/2015 67 Group 6 – Oxygen Family (ns2np4) The Oxygen Family First two members of group – gaseous nonmetallic oxygen (O) & solid nonmetallic sulfur (S) are among most important elements in industry, the environment and living organisms Selenium (Se) & Tellurium (Te) are metalloids Polonium (Po) is radioactive and only metal in the group 1/19/2015 68 Group 6 – Oxygen Family (ns2np4) atomic properties 1/19/2015 physical properties 69 Group 6 – Oxygen Family (ns2np4) Oxygen Family vs Nitrogen Family Groups 5A & 6A have similar Physical & Chemical Properties Oxygen & Nitrogen are low-boiling Diatomic gases Phosphorus (P) & Sulfur (S) occur as polyatomic molecules – P4 & S8 Arsenic (Ar) & Selenium (Se) occur as gray metalloids Antimony (Sb) & Tellurium more more metallic than preceding group members, but display network convalent bonding Bismuth & Polonium are metallic crystals Electrical conductivity increases down group as bonding changes from individual molecules (insulators) to metalloid networks to metallic solids (conductors) 1/19/2015 70 Group 6 – Oxygen Family (ns2np4) Oxygen Family vs Nitrogen Family Allotropism (two or more crystalline or molecular forms of an element) is more common in Group 6A than in Group 5A ● Oxygen has 2 allotropes Dioxygen O2 & Ozone O3 ● Oxygen (O2) gas is colorless, odorless, paramagnetic (unpaired electrons attracted by outside magnetic field), and thermally stable ● Ozone (O3) gas is bluish, has pungent odor, is diamagnetic (paired electrons not affected by external magnetic field), decomposes in heat and Ultraviolet light. ● Ozone in upper atmosphere protects living organisms from Ultraviolet radiation 1/19/2015 71 Group 6 – Oxygen Family (ns2np4) Oxygen Family vs Nitrogen Family Sulfur Allotropes ● 10 forms ● Sulfur bonds to other Sulfur atoms creating rings and chains ● The most stable form is orthorhombic, S8, a crown-shaped ring of 8 atoms 1/19/2015 top view side view 72 Group 6 – Oxygen Family (ns2np4) Oxygen Chemistry vs Nitrogen Chemistry Oxygen & Sulfur occur as anions more often than Nitrogen & Phosphorus Oxygen & Sulfur bond covalently with almost all nonmetals Selenium & Tellurium do some covalent bonding, whereas Polonium behaves like a metal Oxygen has few oxidation states (O2- most common) The other elements in the family exhibit +6. +4, -2 oxidation states, with the +4 state most common in Tellurium and Polonium 1/19/2015 73 Group 6 – Oxygen Family (ns2np4) Oxygen Chemistry vs Nitrogen Chemistry Oxidizing strength of Oxygen is 2nd only to Fluorine The other members of the group behave very little like oxygen being less electronegative and forming anions less often Except for Oxygen, all elements of group 6A form foul smelling, poisonous, gaseous hydrides (H2E) upon treatment of the metal Sulfide, Selenide, etc., with an acid FeSe(s) + HCl (aq) H2Se(g) + FeCl2(aq) 1/19/2015 74 Group 6 – Oxygen Family (ns2np4) Oxygen Chemistry vs Nitrogen Chemistry Bonding & Thermal stability of Group 6A elements have several features in common ● Only Water (H2O) forms Hydrogen bonds, so it melts & boils at higher temperatures than the group 6A H2E compounds (E = S, Se, Te, Po) ● Bond angles drop from the nearly tetrahedral for H2O (104.5o) to around 90o for the Group 6A element hydrides (unhybridized p orbitals) ● E-H bond length increases (bond energy decreases) down group ● Thus, H2Te is stable above 0oC, but H2Po is only stable at extremely cold temperatures; it even decomposes from the heat generated by the radioactivity of the Polonium. 1/19/2015 75 Group 6 – Oxygen Family (ns2np4) Bonding & Thermal Stability (con’t) ● Except for Oxygen, Group 6A elements form a wide range of Halides ● The Halide structure and reactivity patterns depend on the sizes of the central atom and the surrounding Halogens Radius of S < Se < Te < Po Sulfur – Many Fluorides, Few Chlorides, one Bromide As the central atom becomes larger, the Halides become more stable Tetrachlorides & Tetrabromides of Se, Te, Po are known Tetraiodides of Te & Po are known Hexafluorides of S, Se, Te are known 1/19/2015 76 Group 6 – Oxygen Family (ns2np4) ● Halide Structure (con’t) Inverse relationship between bond length & bond strength does not explain pattern of Group 6 Halide formation Crowding of lone electron pairs and Halogen (X) atoms around the central atom With S (small central atom) the larger Halides further down group 7 would be too crowded, which explains why Sulfur Iodides don’t occur 1/19/2015 77 Group 6 – Oxygen Family (ns2np4) ● Halide activity (con’t) Sulfur Tetrafluoride vs Sulfur Hexafluoride SF4 has a lone pair of unshared electrons and empty d orbitals which can be involved in bonding – Highly reactive SF6 uses all of the bonds allowable for S and is tightly packed – chemically inert 1/19/2015 78 Group 6 – Oxygen Family (ns2np4) Highlights of Oxygen Chemistry Most abundant element of the Earth’s surface Many oxides – water, silicates, carbonates, phosphates Most free Oxygen (O2) has biological origin from photosynthesis in algae and multicellular plants Although much more complicated, the basic reaction between oxygen, CO2 and light to form carbohydrates can be represented as: nH2O(l) + nCO2(g) nO2(g) + (CH2O)n The reverse process of combustion and respiration produce CO2 Every element, except He, Ne, Ar (noble gases) form at least one oxide Some oxides have Endothermic heats of reaction, while others have Exothermic ones 1/19/2015 79 Group 6 – Oxygen Family (ns2np4) Highlights of Sulfur Chemistry Two important oxides – SO2 & SO3 ● Sulfur Dioxide (+4 oxidation state) is a colorless choking gas that forms when S, H2S, or a metal sulfide burns in air (oxygen) 2H2S (g) + 3O2(g) 2H2O(g) + 2SO2(g) FeS2(s) + 11O2(g) 2Fe2O3(s) + 8SO2(g) ● Sulfur Dioxide dissolves in water to form Sulfurous acid (H2SO3) – weak acid - which dissociates into an equilibrium solution of hydrated SO2, H+ ions, & Bisulfite (HSO3-) ions SO2(g) + H2O(l) ⇄ [H2SO3(aq)] ⇄ H+(aq) + HSO3-(aq) ● Neither H2SO3 or H2CO3 (both weak acids) can exist as isolated molecules – they dissociate immediately in water 1/19/2015 80 Group 6 – Oxygen Family (ns2np4) ● The S in the Sulfite ion (SO32-) is in the 4+ state and can be easily oxidized to 6+ state. ● Thus, Sulfites are good Reducing Agents ● SO3 (Sulfur Trioxide) is produced by oxidizing SO2 SO2(g) + 1/2O2 V O⇄/K OSO3(g) ● Sulfuric Acid (H2SO4) is a strong acid and the most common industrial chemical It is prepared from SO3, H2O, & conc H2SO4 SO3(g) + conc H2SO4 + H2O H2SO4(l) ● Like other strong acids, sulfuric acid dissociates completely in water forming the Bisulate (HS6+O4)ion ● Conc Sulfuric acid is an excellent dehydrating agent ● The loosely held proton transfer to water in an exothermic formation of Hydronium ion (H3O+) 2 1/19/2015 5 2 81 Group 7 – Halogen Family (ns2np5) Trends in properties down the group are just the opposite of those in Group 1A For Halogens, boiling point, melting point, heats of fusion & vaporization increase down the group The reason for the opposite trends is the different type of bonding: Alkali metals exhibit metallic bonding, which decreases in strength as atoms become larger down group Halogens exist as diatomic molecules that interact through “Dispersion” forces Halogens are quite reactive reacting with metals and nonmetals to form ionic and covalent compounds – metal & nonmetal halide oxides, and oxoacids The halides must gain a single electron to attain the noble gas configuration as a negatively charged anion26 1/19/2015 82 Group 7 – Halogen Family (ns2np5) atomic properties physical properties 1/19/2015 83 Group 7 – Halogen Family (ns2np5) Halogen redox behavior is based on electron affinity, ionic charge density, and electronegativity Halogen higher in group can oxidize halide ion lower in group 1/19/2015 84 Group 7 – Halogen Family (ns2np5) The reactivity of Halogens decreases down group because of the decrease in electronegativity Note: Fluorine is the most electronegative element The F-F bond is the weakest, despite it short length, because the lone pairs of electrons around the first Fluorine atom repel those on the other Fluorine atom weakening the bond Because of the weak bond, F2 reacts with every element, except the noble gases, in many cases explosively The Halogens display the largest range of electronegativity, but all are electronegative enough to behave as nonmetals 1/19/2015 85 Group 7 – Halogen Family (ns2np5) Halogens act as oxidizing agents (they are reduced, gaining electrons) in the majority of their reactions Halogens higher in group oxidize Halide ions lower in the group – Oxidizing ability of X decreases down group F2(g) + 2X-(aq) 2F-(aq) + X2 (X = Cl, Br, I) Reaction of Chlorine with water produces Hypochlorous acid Cl2 + 2H2O(l) ⇄ Cl-(aq) + HClO(aq) + H+ + H2O(l) Chlorination of drinking water (disinfectant) Household bleach is a dilute 5.25% solution of Sodium Hypochlorite (NaClO) Hydrogen Chloride (HCL) is extremely water soluble forming H+ & Cl- ions in solution (Hydrochloric Acid) Hydrochloric Acid occurs in animal stomach fluids and has many industrial uses 1/19/2015 86 Group 7 – Halogen Family (ns2np5) Highlights of Halogen Chemistry The Hydrogen Halides (HX) are formed from the reaction of metal halides and a concentrated acid CaF2(s) + H2SO4(l) CaSO4(s) + 2HF(g) 2NaBr(s) + H3PO4(l) Na3PO4(s) + 3HBr(g) HCl is formed as a byproduct in the chlorination of Hydrocarbons for plastics production CH2=CH2(g) + Cl2(g) ClCH2CH2Cl(g) CH2=CHCl(g) + HCl(g) Ethylene 1,2 Dichloroethane 1-chloroethene Hydrogen Fluoride, with its short, strong bond forms a weak acid (Hydrofluoric acid) with water 1/19/2015 HF(g) + H2O(l) H3O+ + F- 87 Group 7 – Halogen Family (ns2np5) The other Hydrogen Halides (Cl ,Br, I) dissociate completely to form stoichiolmetric amounts of H3O+ ions – strong acids HBr(g) + H2O(l) H3O+(aq) + Br-(aq) Halogens react Exothermically with one another to form many “Interhalogen” compounds ● Diatomic Molecules (ClF, BrCl, IF) The more electronegative atom has the 1- charge; the other less electronegative atom has 1+ charge ● The XYn interhalogens (n = 3,4,5) form when the larger members of the group (X) use “d” orbitals to expand the valence shell The central atom in these molecules has the lower electronegativity and positive charge 1/19/2015 88 Group 7 – Halogen Family (ns2np5) Commercially useful interhalogens include Fluorine compounds used as powerful fluorinating agents, reacting with metals, nonmetals, oxides, and even wood and asbestos Sb(s) + ClF3(l) SnF2(s) + ClF(g) P4(s) + 5ClF3(L) 4PF3(g) + 3ClF(g) The Fluoro Interhalogens react very actively (explosively) with water yielding Hydrogen Fluoride (HF) and the oxoacid. There is no oxidation-reduction reaction and the central atom of the oxoacid retains the same oxidation state 3H2O(l) + Br5+F5(l) 5HF(g) + HBr5+O3 1/19/2015 89 Group 7 – Halogen Family (ns2np5) Oddness & Eveness of Oxidation States ● Odd numbered groups exhibit odd-numbered oxidation states (Na+1, P+5, Cl-1) ● Even numbered groups exhibit even-numbered oxidation states (Ca+2, C+4, O-2) ● Reason: Almost all stable molecules have “Paired” electrons either as bonded or lone pairs ● When bonds form or break, two electrons are involved and the oxidation state changes by “2” Ex. Consider Interhalogens – XY, XY3, XY5, XY7 With Y in the -1 state, the X atoms must be in the +1, +3, +5, +7 state, respectively The X+1 state arises when Y fills its valence shell The X+7 state arises when X is completely oxidized (all electrons shifted to more electronegative Y atom 1/19/2015 90 Group 7 – Halogen Family (ns2np5) Odd Numbered Oxidation States When I2 reacts with F2, Iodine Fluoride (IF) forms I2 + F2 2I+1F-1 Each of the two shared electrons in I2 are used to filled the valence shell of each Fluorine In IF3, Iodine uses two more valence electrons to form two more bonds I+1F- + F2 I+3F3 If only 1 electron changed, then an unstable loneelectron species containing 2 Fluorines would form 1/19/2015 91 Group 7 – Halogen Family (ns2np5) Even Numbered Oxidation States An element in an even-numbered group, such as Sulfur in Group 6A(16), shows the same tendency to have paired electrons in its compounds ● Elemental Sulfur (Ox No = 0) gains or shares 2 electrons to complete its shell ● The Sulfur atom loses 2 electrons to react with Fluorine to form: SF2 (Ox No = + 2) SF2 forms a bent compound. There are a total of 20 valence electrons: 6 by Sulfur, 7 from each Fluorine = 20 total. Eight are placed around the sulfur. Six are placed around each Fluorine. A Fluorine is placed on two sides of the Sulfur. The two unshared electron pairs take up more space than the shared pairs and so the shared pairs move closer together approximately 105 degrees apart. AX2E2 = Tetrahedral Bent, just like water. 92 1/19/2015 Group 7 – Halogen Family (ns2np5) Molecular shapes of the main types of interhalogen ClF3 compounds linear, XY Tshaped XY3 ClF IF7 BrF5 900 Square Pyramidal XY5 Pentagonal Bipyramidal, XY7 1/19/2015 93 Group 7 – Halogen Family (ns2np5) Halogen Oxides Group 7A Halogens form many Oxides that are powerful oxidizing agents (they are reduced by gaining the electrons lost by the oxidized species) The Oxides form Acids with water Dichlorine Monoxide (Cl2O) & Chlorine Dioxide (ClO2) are used to bleach paper 2NaClO3(s) + SO2(g) + H2SO4(aq) 2ClO2(g) + 2NaHSO4(aq) ClO2 has an unpaired electron and the Chlorine (Cl) in the unusual +4 oxidation state (4 electrons are shared with the 2 oxygen atoms, leaving 3 unshaired) 1/19/2015 chlorine dioxide ClO2 94 Group 7 – Halogen Family (ns2np5) Halogen Oxoacids and oxoanions Oxoacids and Oxoanions are formed by reacting the Halogens and their Oxides with Water Most Oxoacids are stable only in solution There are four Oxoacids & Oxoanions The known Halogen Oxoacids Acid Hypochlorous, Chlorous Chloric Perchloric Salt Sodium Hypochlorite, Sodium Chlorite, Sodium Chlorate, Sodium Perchlorate 1/19/2015 95 Group 7 – Halogen Family (ns2np5) Electronegativity of the Halogen Relative strengths of the Halogen Oxoacids depend on two factors ● Electronegativity of the Halogen The more electronegative the halogen, the more electron density it removes from the O-H bond, and the more easily the proton is lost Among Oxoacids with the oxidation state of the Halogen in each Halogen the same, the Acidity (acid strength) decreases as the Halogen’s Electronegativity (EN) decreases Electronegativity – Cl > Br > I Acidity – HOClO2 > HOBrO2 > HOIO2 1/19/2015 96 Group 7 – Halogen Family (ns2np5) ● Oxidation state of the Halogen The oxidation number is a number identical with the valency but with a sign, expressing the nature of the charge of the species in question when formed from the neutral atom The oxidation number of Chlorine in Chlorine Oxoacids Hydrochloric Acid (HCl) - 1 Hypochlorous acid (HOCl) +1 Chlorous Acid (HOCLO or HClO2) + 3 Chloric acid (HClO3 or HOCLO2) + 5 Perchloric acid (HClO4 or HOCLO3) + 7 1/19/2015 97 Group 7 – Halogen Family (ns2np5) ● Oxidation State of the Halogen Among Oxoacids of a given Halogen, such as Chlorine, acid strength decreases as the oxidation state of Halogen decreases The higher the oxidation state (also stated as the number of attached O atoms) of the Halogen, the more electron density it pulls from the O-H bond HOCL+7O3 > HOCL+5O2 > HOCl+3O > HOCl+1 Perchloric Chloric Chlorous Hypochlorous Acid Acid Acid Acid 1/19/2015 98 Group 8 – Noble Gas Family (ns2np6) The Noble gases have completed outer s & p shells Noble gases are generally not reactive – nearly inert Behave more like “Ideal Gases” than any other gases The smallest radii in their period Condense and solidify only at very low temperatures Helium solidifies (with pressure) at -272.2oC (absolute zero is -273.15oC) and boils only 3 degrees higher A few Noble gas compounds have been prepared PtF6 + Xe XePtF6 Other Xenon compounds Xe+2F2 Xe+4F4 Xe+6F6 Xe+8O4 1/19/2015 99 Group 8 – Noble Gas Family (ns2np6) atomic properties 1/19/2015 physical properties 100 Practice Problem What trends exist for Zeff (Effective Nuclear Charge) across a period and down a group Ans: Zeff, the effective nuclear charge In multi-electron atoms an electron feels the attraction from the positively charged nucleus (protons) and the repulsion of like-charged electrons Electron repulsion shields the electron from the nuclear attraction making the electron easier to remove Shielding reduces the “full nuclear charge” to an effective nuclear charge (Zeff) Zeff increases across a period and decreases down a group 1/19/2015 101 Practice Problem How does the Effective Nuclear Charge, Zeff , influence atomic size, Ionization Energy (IE), and Electronegativity (EN)? Ans: As you move to the right across a Period: The Atomic Size decreases The Ionization Energy increases The Electronegativity increases All because of the increased Zeff 1/19/2015 102 Practice Problem How are covalent and metallic bonding similar? Ans: Covalent and Metallic bonding involve sharing of electrons between atoms Covalent bonding includes sharing between a small number of atoms (usually two) Metallic bonding involves essentially all the atoms in a given sample 1/19/2015 103 Practice Problem Which of following pairs react to form covalent compounds, ionic compounds? a. Be & C b. Sr & O Ans: a. Covalent 1/19/2015 c. Ca & Cl d. P & F (2 non-metals) b. Ionic (Oxygen is most ionic in group 6) c. Ionic Metal & non-metal) d. Covalent (2 non-metals) 104 Practice Problem Which member of each pair gives the more acidic solution? a. CO2 or SrO b. SnO or SnO2 c. Cl2O or Na2O d. SO2 or MgO Ans: a. Carbon dioxide will form a more acidic solution 2 CO2(g) + H2O(l) → H2CO3(aq) (Weak acid) SrO(s) + H2O(l) → Sr(OH)2(aq) (Sr oxides form basic solutions) b. Tin(IV) oxide (SnO) An element with more than 1 oxidation state exhibits less metallic behavior in its higher state – more acidic c. Dichlorine Oxide (Cl2O) Non-metal oxides form acidic solution; metallic oxides form basic solution d. Sulfur Dioxide (SO2) Non-metal oxides for acidic solution; metallic oxides form basic solution 1/19/2015 105 Practice Problem Each of the following properties shows a regular trend in Group 1A. Predict whether each increases or decreases up the group a. Melting Point b. E – E bond length c. Hardness d. Molar Volume e. Lattice Energy of E-Br Ans: 1/19/2015 a. Increases Increased Lattice Energy b. Decreases Decreasing radius of atom c. Increases Increased Lattice Energy d. Decreases Decreasing radius of atom e. Increases The atomic radius decreases as you move up a group increasing the lattice energy. 106 Practice Problem The melting points of Alkaline Earth metals (group 2A) are many times higher than the Alkali metals (group 1A) Explain this difference on the basis of atomic properties Ans: Metal atoms are held together by metallic bonding, a sharing of valence electrons. Alkaline earth metal atoms have one more valence electron than alkali metal atoms, so the number of electrons shared is greater Thus, metallic bonds in alkaline earth metals are stronger than in alkali metals. Melting requires overcoming the metallic bonds To overcome the stronger alkaline earth metal bonds requires more energy (higher temperature) than to overcome the alkali earth metal bonds. 1/19/2015 107 Practice Problem Many compounds of group 3A elements have chemical behavior that reflects an electron deficiency Explain electron deficiency with illustrative reactions Ans: Compounds of Group 3A(13) elements (ns2np1), like Boron (B), have only six electrons in their valence shell when combined with Halogens to form three bonds Having six electrons, rather than an octet, results in an “electron deficiency,” i.e., violates octet rule As an electron deficient central atom, Born (B) is trigonal planar (AX3). Upon accepting an electron pair to form a bond, the shape changes to tetrahedral (AX4) BF3(g) + NH3(g) → F3B–NH3(g) F AX3 F B F 1/19/2015 AX4 108 Practice Problem Nearly every compound of Silicon (Group 4A) has the element in the +4 oxidation state. In contrast, most compounds of Lead have the element in the +2 state a. What general observation does this fact illustrate? Ans: The increased stability of the lower oxidation state as one goes down a group b. Explain in terms of atomic structure and molecular properties Ans: As the atoms become larger (Pb > Si), the strength of the bonds to other elements becomes weaker, and insufficient energy is gained in forming the bonds to offset the additional ionization or promotion energy c. Give an analogous example from Group 3A Ans: Thallium(Tl+) is more stable than Tl3+, but Al3+ is the only table oxidation state for Al 1/19/2015 109 Practice Problem Based on the relative sizes of Fluorine (F) and Chlorine(Cl), predict the structure of PF2Cl3 Ans: From the Lewis structure, the Phosphorus (Central atom) has 5 electron groups for a trigonal bipyramidal molecular shape In this shape, the three groups in the equatorial plane have greater bond angles (120°) than the two groups above and below this plane (90°) The Chlorine atoms (larger than Fluorine atoms) would occupy the planar sites where there is more space for the larger atoms 1/19/2015 110 Practice Probem A Halogen (X2) disproportionates (one X is reduced and one is oxidized) in base in several steps to X- and XO-3. Write the overall reaction for the disproportionation of Br2 to Br- and BrO3-1 Ans: A substance that disproportionates serves as both an oxidizing and reducing agent. Assume that OH– serves as the base Write the reactants and products of the reaction, and balance like a redox reaction Br2(l) + OH– (aq) → Br– (aq) + BrO3-1 (aq) + H2O(l) Br2(l) + 6 OH– (aq) → Br– (aq) + BrO3-1 (aq) + 3 H2O(l) Balance e- on each side using coefficients 0 5 x -1 = -5 +5 3 Br2(l) + 6 OH– (aq) → 5 Br– (aq) + BrO3-1 (aq) + 3 H2O(l) 1/19/2015 111 Practice Problem The main reason Alkali metal dihydrides (MX2) do not form is the Ionization Energy (IE) of the metal Why is the IE so high for alkali metals Ans: Alkali metals have an outer electron configuration of ns1 The first electron lost by the metal is the ns electron, giving the metal a noble gas configuration Second ionization energies for alkali metals are high because the electron being removed is from the next lower energy level and electrons in a lower level are more tightly held by the nucleus. The metal would also lose its noble gas configuration 1/19/2015 112