* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CTL - Molecular Immunology
Complement system wikipedia , lookup
Immunocontraception wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Human leukocyte antigen wikipedia , lookup
Immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Major histocompatibility complex wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
DNA vaccination wikipedia , lookup
Duffy antigen system wikipedia , lookup
Adaptive immune system wikipedia , lookup
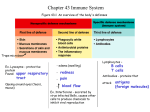
T cells do not recognise native antigens Y Y Y Y Y Y Y Cross-linking of surface membrane Ig YYY Y Y Y Y Y B B B B B BB B B Proliferation and antibody production T T No proliferation No cytokine release Y Y Antigens must be processed in order to be recognised by T cells T Y Soluble native Ag Cell surface native Ag Soluble peptides of Ag Cell surface peptides of Ag presented by cells that express MHC antigens Cell surface peptides of Ag ANTIGEN PROCESSING No T cell response No T cell response No T cell response No T cell response T cell response Early evidence that antigens are catabolised M Macrophages and radiolabelled Listeria monocytogenes M Internalisation M Rapid binding to cell surface Degradation M of bacteria and release of Radiolabelled protein into supernatant and cells How is antigen catabolism linked to T cell proliferation? The interaction of T cells with macrophages requires antigen catabolism NO T CELLS BIND Listeria-specific T cells Listeria coated plastic T Listeria NO T CELLS NO T CELLS NO T CELLS BIND BIND BIND M 0mins M M T CELLS BIND M 60mins T cell do not bind stably to antigen presenting cells unless the antigen is catabolised Only metabolically active cells can process antigen Listeriaspecific T cells M Fix with paraformaldehyde or poison with sodium azide M Pulse with Listeria for 60min & wash cells T M Add Listeria specific T cells NO T CELLS BIND Determinants recognised by T cells are generated by catabolic activity that is dependent upon the viability of macrophages Antigen presenting cells must be viable to PROCESS antigen Antigen presentation does not require metabolically-active cells Listeria M M M M Fix with paraformaldehyde or poison with sodium azide Add Listeria specific T cells T T CELLS BIND M Antigen presenting cells do not need to be viable to PRESENT antigen Where does antigen processing take place? Add Listeria specific T cells Listeria M M M T M T CELLS BIND Listeria Incubate with CHLOROQUINE M M M M NO T CELLS BIND Chloroquine inhibits lysosomal function (a lysosomotrophic drug) Antigen processing involves the lysosomal system What form of antigen is produced by antigen processing? T Ovalbumin specific T cell line Digested ovalbumin Native ovalbumin Ag APC T cell response APC APC APC APC Viable Fixed Viable Fixed T T T T T T T T T T T T T T T T Catabolism reduces antigens to peptides that can be recognised by T cells Summary of exogenous antigen processing • T cells can not recognise native antigens • Antigens must be processed for recognition by T cells • Antigens catabolism occurs inside cells • Only metabolically active cells can process antigen • Antigen presentation does not require metabolically-active cells • Antigen processing involves the lysosomal system • Catabolism reduces antigens to peptides • Because extracellular antigens are dealt with by the lysosomal system, lysosomal antigen processing is part of the EXOGENOUS antigen processing pathway Is exogenous antigen processing sufficient? • Macrophages have welldeveloped lysosomal systems M • Specialised for motility, phagocytosis and the introduction of particles to the lysosomal system Most cell types do not have lysosomal systems developed as well as macrophages BUT Viruses can infect most cell types A non-lysosomal mechanism to process antigens for presentation to T cells is required Infectious viruses raise CTL that recognise antigens that are not generated by the exogenous pathway Infectious influenza Strong T cell response CTL CTL CTL CTL Cloned anti- CTL assay Kill CTL CTL CTL CTL CTL CTL CTL CTL CTL CTL CTL CTL CTL No treatment Kill + Chloroquine Lysosome inhibitors do not inhibit the generation of antigens recognised by most CTL Most CTL do not recognise lysosomally-derived antigens Inactive viruses raise CTL to antigens that are generated by the exogenous pathway Inactivated influenza CTL assay CTL Weak T cell response Cloned anti- Kill CTL No treatment CTL CTL CTL CTL CTL CTL CTL CTL CTL CTL CTL No Kill + Chloroquine Lysosomal inhibitors inhibit the generation of antigens from INACTIVE virus Some CTL can recognise lysosomally-derived antigens Non-lysosomal processing The antigens of infectious & inactivated viruses are clearly generated by different mechanisms Infectious viruses use cellular protein synthesis machinery to replicate Inactivated viruses do not synthesise protein CTL raised with infectious virus CTL raised with non-infectious virus CTL CTL Untreated Protein synthesis inhibitor-treated Protein synthesis is required for virus infected target cells to express antigens recognised by CTL Non-lysosomal antigen processing Inactive virus raises a weak CTL response The processing of antigens from inactive viruses is sensitive to lysosomotrophic drugs ANTIGENS FROM INACTIVE VIRUSES ARE PROCESSED VIA THE EXOGENOUS PATHWAY Infectious virus raises a strong CTL response The processing of antigens from infectious viruses is NOT sensitive to lysosomotrophic drugs Most CTL recognise antigens generated via a non-lysosomal pathway Protein synthesis is required for non-lysosomal antigen processing ANTIGENS FROM INFECTIOUS VIRUSES ARE PROCESSED VIA THE ENDOGENOUS PATHWAY Do the two pathways generate the same type of T cell receptor ligand? Endogenous antigen processing also generates peptides Influenza virus CTL Nucleoprotein CTL Peptides of nucleoprotein CTL Infectious virus sensitises for lysis Native antigen fails to sensitise for lysis Synthetic peptide antigens sensitise targets for lysis Protein/antigen synthesis No protein/antigen synthesis No protein/antigen synthesis but peptides are pre-formed The site of pathogen replication or mechanism of antigen uptake determines the antigen processing pathway used Y EXTRACELLULAR OR ENDOSOMAL REPLICATION Vesicular Compartment Contiguous with extracellular fluid Exogenous processing (Streptococcal, Mycobacterial antigens) INTRACELLULAR REPLICATION Cytosolic compartment Endogenous processing (Viral antigens) Distinct mechanisms of antigen generation are used to raise T cells suited to the elimination of endogenous or exogenous pathogens Y Antigens generated by endogenous and exogenous antigen processing activate different effector functions EXOGENOUS PATHOGENS Eliminated by: ENDOGENOUS PATHOGENS Eliminated by: Antibodies and phagocyte activation by T helper cells that use antigens generated by EXOGENOUS PROCESSING Killing of infected cells by CTL that use antigens generated by ENDOGENOUS PROCESSING Stages of endogenous and exogenous antigen processing UPTAKE Access of native antigens and pathogens to intracellular pathways of degradation DEGRADATION Limited proteolysis of antigens to peptides ANTIGEN-MHC COMPLEX FORMATION Loading of peptides onto MHC molecules ANTIGEN PRESENTATION Transport and expression of peptide-MHC complexes on the surface of cells for recognition by T cells Uptake of exogenous antigens Membrane Ig receptor mediated uptake Y Phagocytosis Complement receptor mediated phagocytosis Pinocytosis Y Fc receptor mediated phagocytosis Uptake mechanisms direct antigen into intracellular vesicles for exogenous antigen processing Receptor-mediated uptake enhances the efficiency of the T cell response Receptor-mediated antigen uptake Non-receptor -mediated uptake 100 % of max. T cell response 75 50 25 0 10-3 10-2 10-1 Antigen gml-1 Exogenous pathway Cell surface Uptake Protein antigens In endosome Endosomes Increase in acidity To lysosomes Cathepsin B, D and L proteases are activated by the decrease in pH Proteases produce ~24 amino acid long peptides from antigens Drugs that raise the pH of endosomes inhibit antigen processing MHC class II maturation and invariant chain In the endoplasmic reticulum Invariant chain stabilises MHC class Need to prevent newly II by non- covalently binding to the synthesised, unfolded self proteins from binding immature MHC class II molecule and forming a nonomeric complex to immature MHC Invariant chain CLIP peptide and b chains of MHC class II molecules CLIP A peptide of the invariant chain blocks the MHC molecule binding site. This peptide is called the CLass II associated Invariant chain Peptide (CLIP) Class II associated invariant chain peptide (CLIP) Cell surface Uptake (binv)3 complexes directed towards endosomes by invariant chain Endosomes Cathepsin L degrades Invariant chain CLIP blocks groove in MHC molecule MHC Class II containing vesicles fuse with antigen containing vesicles Removal of CLIP ? How can the peptide stably bind to a floppy binding site? Competition between large number of peptides HLA-DM assists in the removal of CLIP HLA-DM HLA-DR HLA-DM: Crystallised without a peptide in the groove In space filling models the groove is very small HLA-DM Single pocket in “groove” insufficient to accommodate a peptide HLA-DR Multiple pockets in groove sufficient to accommodate a peptide HLA-DM catalyses the removal of CLIP HLA-DM Replaces CLIP with a peptide antigen using a catalytic mechanism (i.e. efficient at substoichiometric levels) Discovered using mutant cell lines that failed to present antigen HLA-DR HLA-DM MIIC compartment HLA-DO may also play a role in regulating DM Sequence in cytoplasmic tail retains HLA-DM in endosomes Surface expression of MHC class IIpeptide complexes Exported to the cell surface (t1/2 = 50hr) Sent to lysosomes for degradation MIIC compartment sorts peptide-MHC complexes for surface expression or lysosomal degradation Endogenous antigen processing UPTAKE Antigens/pathogens already present in cell DEGRADATION Antigens synthesised in the cytoplasm undergo limited proteolytic degradation in the cytoplasm ANTIGEN-MHC COMPLEX FORMATION Loading of peptide antigens onto MHC class I molecules is different to the loading of MHC class II molecules PRESENTATION Transport and expression of antigen-MHC complexes on the surface of cells for recognition by T cells Degradation in the proteasome Cytoplasmic cellular proteins, including non-self proteins are degraded continuously by a multicatalytic protease of 28 subunits The components of the proteasome include MECL-1, LMP2, LMP7 These components are induced by IFN- and replace constitutive components to confer proteolytic properties. LMP2 & 7 encoded in the MHC Proteasome cleaves proteins after hydrophobic and basic amino acids and releases peptides into the cytoplasm Peptide antigens produced in the cytoplasm are physically separated from newly formed MHC class I ENDOPLASMIC RETICULUM Newly synthesised MHC class I molecules CYTOSOL Peptides need access to the ER in order to be loaded onto MHC class I molecules Transporters associated with antigen processing (TAP1 & 2) Hydrophobic transmembrane domain Lumen of ER Peptide ER membrane Cytosol Peptide Peptide Peptide antigens from proteasome ATP-binding cassette (ABC) domain Transporter has preference for >8 amino acid peptides with hydrophobic C termini. Discovery of the role of TAP1 & TAP2 in antigen processing Normal antigen presenting cell line with stable surface MHC class I expression Chemically-induced mutant antigen presenting cell line with unstable (floppy) MHC class I expressed intracellularly Analysis of genes in the MHC of the mutant cell line showed mutations in a pair of ABC transporter genes X √ Transfection of normal TAP genes into mutant APC restored stable surface MHC class I expression Mutations in TAP genes affect the supply of peptides to the ER MHC class I stability is dependent upon a supply of peptides Maturation and loading of MHC class I Peptide Peptide Peptide Endoplasmic reticulum B2-M Calnexin binds binds and to nascent stabilises class I chain floppy until b2-M binds MHC Tapasin, calreticulin, TAP 1 & 2 form a complex with the floppy MHC Cytoplasmic peptides are loaded onto the MHC molecule and the structure becomes compact Fate of MHC class I Exported to the cell surface Sent to lysosomes for degradation Evasion of immunity by interference with endogenous antigen processing Peptide Peptide Endoplasmic reticulum HSV protein blocks transport of viral peptides into ER Sent to lysosomes for degradation Evasion of immunity by interference with endogenous antigen processing Normally exported to the cell surface Adenoviral protein retains MHC class I in the ER Sent to lysosomes for degradation Summary • T and B cells recognise antigen differently • Antigen must be catabolised before T cells can recognise it • Antigen processing generates antigenic peptides • Exogenous antigen processing takes place in lysosomes • Endogenous processing is non-lysosomal • The mechanism of antigen processing depends upon the compartment in which the pathogen replicates • Endogenous and exogenous antigen processing both involve uptake, degradation, complex formation and presentation • Exogenous antigen processing uses invariant chain and HLA-DM • Endogenous antigen processing uses proteasomes and peptide transporters in antigen processing • Pathogens can evade immunity by disrupting antigen processing