* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download UNIT-1 Carbohydrates
Point mutation wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Electron transport chain wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Proteolysis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Genetic code wikipedia , lookup
Metalloprotein wikipedia , lookup
Butyric acid wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
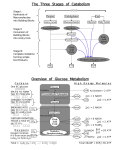
UNIT‐1 Carbohydrates • All of these molecules exist as small, single units generally called monomers, which can then be combined to form larger units called polymers Molecules often have functional groups. Functional groups give different characteristics to molecules. R - NH2 – amino group R - OH – hydroxyl group R – COOH – carboxyl or carboxylic acid group R = the rest of the molecule ? As we look at molecules, which molecules have these functional groups? R - NH2 – amino group R - OH – hydroxyl group R – COOH – carboxyl or carboxylic acid group R = the rest of the molecule How do monomers become polymers? Amino acid (monomer) Amino acid (monomer) Polypeptide or protein polymer How do monomers become polymers? Dehydration synthesis – Removal of a water molecule between two reacting molecules forming a new covalent bond in the process How do polymers become monomers? Hydrolysis – Addition of a water molecule to a polymer to break a bond within a polymer to form the monomers Molecule Monomer Polymer Carbohydrates Monosaccharide Polysaccharide or Carbohydrate Protein Amino Acid Polypeptide or Protein Nucleic Acid Nucleotide Nucleic Acid CARBOHYDRATES Function: quick energy structural support Characteristics: H – C – OH ratio of hydrogen to oxygen atoms is 2:1 Monomer is the monosaccharide What do these three carbohydrates have in common? What is different between them? Starch is a polymer of glucose used for storage. It is found in plants. Cellulose is a polymer of glucose - used for storage. It is found in plants. We cannot digest cellulose! Citric acid cycle Energetics • Energy is conserved in the reduced coenzymes NADH, FADH2 and one GTP • NADH, FADH2 can be oxidized to produce ATP by oxidative phosphorylation acetyl CoA 2 ADP +1.5 2 Pi 1.5 TCA ETS 1.5 2 ATP 3 NAD+ FADH2 FAD 7.59 ATP ETS 3 NADH 9 Pi 7.59 ADP +7.5 ATP generated by the cycle 3 NAD+ 3 NADH ETS 3*2.5=7.5 ATP FAD FADH2 ETS 1.5 ATP Substrate level phosphorylation 1 GTP 10 ATP Equivalents Total Regulation of the TCA Cycle • Citrate synthase ‐ regulated by availability of substrates ‐ acetyl‐CoA and oxaloacetate, citrate is a competitive inhibitor; Allosteric: ‐ NADH , ATP,succinyl‐CoA • Isocitrate dehydrogenase – NADH,ATP inhibit, ADP and NAD+ Ca++ activate • α ‐Ketoglutarate dehydrogenase ‐ NADH and succinyl‐CoA inhibit, AMP Ca++activate Glycolysis