* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The ingredients of life. - Waterford Public Schools
Basal metabolic rate wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Genetic code wikipedia , lookup
Isotopic labeling wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Carbon sink wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Photosynthesis wikipedia , lookup
Biosequestration wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
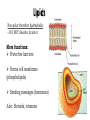
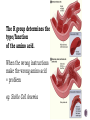
“The ingredients of life.” You = collection of organic molecules! McDonald’s Total calories: 2010 Panera Bread Total calories: 2160 Subway Total calories: 2010 Starbucks Total calories: 2030 Carbohydrates Protein Fat Organic compounds all contain… carbon! Carbon is special. It’s atomic properties cause it to easily bond with lots of other atoms and molecules. Carbon atoms love to form strong bonds to other carbon atoms, creating chains and rings. Life is IMPOSSIBLE without carbon. “Large Molecule” Made from carbon compounds Monomers: small subunits or “building blocks” Polymers: large units composed of multiple monomers Mono- = one Poly- = many Monomer Polymer Each macromolecule performs it’s own tasks. The basic structure and function of each type of macromolecule is similar in all organisms (from the simplest bacteria to complex humans) Made of carbon, hydrogen, and oxygen (CHO) Monomer(s): Monosaccharides/Disaccharides Glucose Fructose – in fruits Galactose – in milk (Two monosaccharides Together) Sucrose - table sugar Lactose – in milk aka “simple sugars” Polymer: Polysaccharides aka “complex carbohydrate” Plant/animal’s way of “storing” simple sugars Starch – in plants Cellulose – in plants Glycogen – in animals … Polysaccharides Starch - digestible (rice, wheat, potatoes etc) Cellulose – not digestible (wood, paper) Glycogen – stored in liver for when blood glucose (“blood sugar”) runs low Function: Source of ENERGY! Quick energy (glucose) Short-term storage of quick energy (starch and glycogen) Made of carbon, hydrogen, and (some) oxygen Monomers: Glycerol + Fatty Acid Polymer: Triglyceride Function #1: Long term energy storage Video of oil spill Non-polar therefore hydrophobic – DO NOT dissolve in water More functions: Protective barriers Forms cell membrane (phospholipids) Sending messages (hormones) Also: Steroids, vitamins Made of carbon, hydrogen, oxygen, nitrogen(CHON) Monomer: Amino Acids Polymer: Polypeptides Function: Structural support (collagen) Transport (hemoglobin) Defense (antibodies) Enzymes (amylase) The type of amcino acid is determined by it’s “R” group 20 amino acids in total 8 “essential” amino acidsWe cannot make them ourselves Only “R” group changes The R group determines the type/function of the amino acid. When the wrong instructions make the wrong amino acid = problem eg. Sickle Cell Anemia Made of carbon, hydrogen, oxygen, nitrogen, phosphorus (CHONP) Monomer: Polymer: Nucleotide Nucleic acid (DNA/RNA) Function: Stores/transmits genetic information Build proteins DNA AND RNA. That’s it.