* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Serine Residues 286, 288, and 293 within the CIITA: A
Hedgehog signaling pathway wikipedia , lookup
Cellular differentiation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Signal transduction wikipedia , lookup
Major histocompatibility complex wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
List of types of proteins wikipedia , lookup
Cell nucleus wikipedia , lookup
VLDL receptor wikipedia , lookup
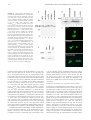
The Journal of Immunology Serine Residues 286, 288, and 293 within the CIITA: A Mechanism for Down-Regulating CIITA Activity through Phosphorylation1 Susanna F. Greer,* Jonathan A. Harton,* Michael W. Linhoff,* Christin A. Janczak,† Jenny P.-Y. Ting,2* and Drew E. Cressman2,3† CIITA is the primary factor activating the expression of the class II MHC genes necessary for the exogenous pathway of Ag processing and presentation. Strict control of CIITA is necessary to regulate MHC class II gene expression and induction of an immune response. We show in this study that the nuclear localized form of CIITA is a predominantly phosphorylated form of the protein, whereas cytoplasmic CIITA is predominantly unphosphorylated. Novel phosphorylation sites were determined to be located within a region that contains serine residues 286, 288, and 293. Double mutations of these residues increased nuclear CIITA, indicating that these sites are not required for nuclear import. CIITA-bearing mutations of these serine residues significantly increased endogenous MHC class II expression, but did not significantly enhance trans-activation from a MHC class II promoter, indicating that these phosphorylation sites may be important for gene activation from intact chromatin rather than artificial plasmid-based promoters. These data suggest a model for CIITA function in which phosphorylation of these specific sites in CIITA in the nucleus serves to down-regulate CIITA activity. The Journal of Immunology, 2004, 173: 376 –383. T he major histocompatibility CIITA plays a pivotal role in initiating immune responses by activating the expression of the MHC class II genes and associated molecules (1– 6). MHC class II proteins facilitate the presentation of exogenously derived antigenic peptides on the surface of APCs, leading to recognition by CD4⫹ T cells and subsequent activation of the cellular components of the immune system. Expression of MHC class II genes is constitutive on B cells, monocytes, macrophages, and dendritic cells and is inducible on most other cell types in response to cytokines such as IFN-␥ and TNF-␣. Both constitutive and inducible expressions of these genes require CIITA, whereas loss of CIITA results in a severe immunodeficiency characterized by complete loss of MHC class II-mediated Ag presentation (7, 8). Within the nucleus, CIITA interacts with a variety of transcription factors and coactivators such as regulatory factor X (RFX)5, NF-Y, CREB, and CREB binding protein at the promoter regions of target genes (9 –14). CIITA acts as a molecular scaffold to stabilize the interactions of these requisite MHC class II transcription factors with the conserved MHC class II promoter motifs X1, X2, and Y, and is therefore critical for the formation of the MHC class II enhanceosome complex. Structure-function studies have dem*Lineberger Comprehensive Cancer Center and Department of Microbiology and Immunology, University of North Carolina, Chapel Hill, NC 27599; and †Department of Biology, Sarah Lawrence College, Bronxville, NY 10708 Received for publication March 4, 2004. Accepted for publication April 19, 2004. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 1 This work was supported by grants from the National Institutes of Health (AI29564, AI45580, and AI41751; to J.P.-Y.T.), a National Multiple Sclerosis Society Career Transition Award (to S.F.G.), an American Cancer Society fellowship (to J.A.H.), and a Milton J. Petrie-Irvington Institute fellowship and the National Science Foundation (MCB0212067; to D.E.C.). onstrated that although CIITA itself does not bind DNA, it contains a potent trans-activation domain in the N terminus (15, 16). CIITA also contains a variety of other recognizable motifs, including proline-serine-threonine-rich domains (17), a GTP-binding domain (GBD)4 (18), and three nuclear localization signals (NLSs) (19 –22). Loss of GTP binding prevents translocation of CIITA into the nucleus. Although the GBD is not an NLS, binding of GTP to this region probably causes a conformational change, permitting nuclear translocation (18). Once in the nucleus, CIITA activates the expression of a variety of genes, leading to MHC class II Ag surface presentation (5, 6). The precise mechanisms regulating CIITA activity and MHC class II expression have been areas of recent intense interest. It has previously been shown that CIITA activity is inducible by IFN-␥, enhanced through association with mono-ubiquitin, and down-regulated by the actions of histone deacetylases 1 and 2 (23–25). Although CIITA acts primarily in the nucleus, the CIITA protein has been found to localize to both the cytoplasm and nucleus and to undergo nuclear import (19 –21, 26). The purpose of this dual subcellular localization remains to be determined. Treatment of cells with leptomycin B, an inhibitor of nuclear export, causes accumulation of CIITA in the nucleus. Export of CIITA is probably mediated by one or more nuclear export signals and has recently been shown to be regulated in part by the GTP-binding domain (27). The possibility therefore remains that nuclear export of CIITA plays a role in down-regulating its activity and MHC class II transcription. One mechanism often involved in regulating both the subcellular localization and the activation of proteins is phosphorylation. Phosphorylation of components of the nuclear transport machinery globally down-regulates nuclear import, but does not affect nuclear export (28). Additionally, phosphorylation of specific proteins, including cyclin B1 (29), NF-AT (30), and the yeast transcription 2 J.P.-Y.T. and D.E.C. contributed equally to this paper and should be considered co-senior authors. 3 Address correspondence and reprint requests to Dr. Drew E. Cressman, Department of Biology, Sarah Lawrence College, 1 Mead Way, Bronxville, NY 10708. E-mail address: [email protected] Copyright © 2004 by The American Association of Immunologists, Inc. 4 Abbreviations used in this paper: GBD, GTP-binding domain; NLS, nuclear localization signal; PKA, protein kinase A; RFX, regulatory factor X; TFIIB, transcription factor class II B. 0022-1767/04/$02.00 The Journal of Immunology factor Pho4 (31), can alter their subcellular localization. We previously showed that CIITA is phosphorylated on a variety of different serine residues, including a serine at aa 286 (32). Recently, additional phosphorylation sites have been assigned to the general region between aa 253–321 using deletion mutants. Although these specific mutants were not tested for transcriptional activity, a separate experiment in that paper showed that the treatment of cells with inhibitors of phosphatase activity resulted in an increase in CIITA trans-activation and that this is associated with an increase in CIITA oligomerization and nuclear accumulation (33). Another paper mapped a protein kinase A (PKA) phosphorylation domain to the region between residues 325– 408, with four serines identified as the PKA targets. Mutations of those four serines caused only a modest (2-fold or less) effect on the CIITA trans-activation function (34). Finally, a report showed that constitutively active protein kinase C increased CIITA expression, whereas a dominant negative mutant of protein kinase C blocked IFN-␥-induced MHC class II gene expression, although the phosphorylation sites were not mapped (35). Hence, there appears to be a complex array of phosphorylation targets. However, the functional consequence of phosphorylation on specific residues is less clear and represents a focus of this report. In this study we show that CIITA found in the nucleus, but not the cytoplasm, is predominantly phosphorylated, and we identify serines 286, 288, and 293 as phosphorylation targets. These findings largely agree with and extend the findings of the previous report (36). Surprisingly, mutation of S293 together with S288 or S286 significantly enhanced the nuclear translocation of CIITA and its ability to trans-activate the endogenous expression of MHC class II mRNA, indicating that whereas phosphorylation of these residues is not required for nuclear import, it is important for mediating the subsequent decrease in endogenous CIITA trans-activation ability. Together these findings suggest that CIITA function is down-regulated by phosphorylation at these critical residues. Materials and Methods Plasmids and reagents All mutants were constructed using the pCDNA3.FLAG.CIITA parent vector containing an eight-amino acid FLAG epitope (DYKDDDDK) upstream of the first methionine of CIITA (17). C-terminal CIITA deletion mutants were constructed by QuikChange site-directed mutagenesis (Stratagene, La Jolla, CA), resulting in a stop codon placed at the indicated position within the CIITA sequence. Stop mutations were made at codons for aa 187, 225, 261, 271, 283, 294, 307, and 313. CIITA containing the internal deletion of aa 132–302 (CIITA⌬132–301) was constructed as described previously (17). CIITA⌬1–335 was made by introducing a BglII mutation at residue 334, then subcloning residues 335-1130 into BamHI/ EcoRI restriction sites in the pCDNA3-His C vector (Invitrogen, Carlsbad, CA). Point mutations of serine residues to alanine at aa 286, 288, and 293 were introduced by QuikChange site-directed mutagenesis. Double and triple mutants were generated by sequential mutagenic reactions. CIITANLS and CIITA-5aa have been described previously (19). All constructs were verified by sequence analysis. In vitro transcription/translation of wild-type CIITA was completed with TNT T7 Quick Master Mix (Promega, Madison, WI) supplemented with 0.5 g of plasmid DNA template and 40 M methionine. The reaction was incubated at 30°C for 60 min and prepared for gel analysis. Where indicated, extracts were treated with 40 U/l protein phosphatase (New England Biolabs, Beverley, MA) at 30°C for 1 h. Immunofluorescence FLAG-tagged wild-type CIITA (1 g) or the indicated CIITA mutants were transfected into COS-7 cells growing in two-well chamber slides and assayed for subcellular localization. Where indicated, 2.5 g/ml actinomycin D (Sigma-Aldrich, St. Louis, MO) were added to the cells 3 h before harvesting. At 24 h post-transfection, slides were rinsed in PBS, fixed in acetone/PBS (3/2 dilution) for 4 min, incubated 1 h with M5 anti-FLAG mouse mAb (Sigma-Aldrich) diluted 1/500 in PBS/1% BSA, rinsed, incubated 1 h with FITC-conjugated goat anti-mouse secondary Ab (1/500 377 dilution; BD Pharmingen, San Diego, CA), then rinsed and mounted in Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA), and observed at ⫻50 magnification. Cell culture and luciferase assays HeLa human epitheloid carcinoma and COS-7 monkey kidney fibroblast cell lines were grown in DMEM supplemented with 10% FCS, 300 mg/L L-glutamine, and 1% penicillin/streptomycin. Raji human lymphoma cells were grown in RPMI 1640 supplemented as described above. All cells were cultured at 37°C in 5% CO2. All transfections were performed using FuGene 6 (Roche, Indianapolis, IN) according to the instructions of the manufacturer. Cells were split to six-well plates 12 h before transfection. One microgram of each plasmid was transfected into cells along with 1 g of the class II DR promoter fused to a luciferase reporter, and cells were harvested at 24 h post-transfection in 200 l of 1⫻ reporter lysis buffer (Promega). Forty microliters were assayed for luciferase activity as previously described (36). The reporter plasmid for luciferase experiments contained ⬃300 bp of the DRA promoter (37) fused upstream of the luciferase gene in the pGL2-Basic vector (Promega). Extracts were normalized to protein concentration, which was determined to be equivalent to normalization by cotransfected -galactosidase gene assays (data not shown). Each transfection was performed in triplicate. Cellular fractionation and immunoblots Cells were transfected in six-well plates with the indicated plasmids, harvested at 24 h post-transfection, and fractionated into whole-cell, cytoplasmic, or nuclear extracts. Whole-cell extracts were prepared by lysis of cells in 400 l of RIPA buffer (10 mM Tris (pH 7.4), 2% deoxycholate, 1% Nonidet P-40, 150 mM NaCl, 0.1% SDS, 2 mM EDTA, 50 mM NaF, and 1⫻ protease inhibitors (Roche)). Cytoplasmic and nuclear extracts were prepared as described previously (38). Protein concentrations of each fraction were determined by the Bradford method. For immunoblot analyses, 10 g of each fraction was separated on 5% SDS-polyacrylamide gels, transferred to nitrocellulose, and blotted with 2 g of anti-FLAG M5 Ab (Sigma-Aldrich). Anti-transcription factor class II B (TFIIB) Abs (Santa Cruz Biotechnology, Santa Cruz, CA) were used to blot for integrity of the extracts. For immunoprecipitation studies, whole-cell extracts were prepared in RIPA buffer as described above. Extracts were incubated at 4°C overnight with 2 g of anti-FLAG or anti-Myc Ab plus 20 l of Dynabeads (Dynal Biotech, Great Neck, NY) per reaction. Beads were washed three times with RIPA buffer, boiled in 2⫻ loading buffer with SDS, and loaded onto 5% SDS-polyacrylamide gels. Real-time PCR cDNA was synthesized as described previously (39). Real-time PCR was performed using the ABI PRISM 7900 sequence detection system (PerkinElmer, Foster City, CA). MHC class II probes were labeled at the 5⬘ end with the reporter dye FAM and at the 3⬘ end with the quencher dye TAMRA. The 18S rRNA probe was labeled at the 5⬘ end with the reporter dye TET and at the 3⬘ end with the quencher dye TAMRA. Primer and probe sequences were previously reported (25), and real-time PCR analysis of cDNA products were conducted as previously described (39). Values were calculated based on standard curves generated for each gene. Normalization of samples was determined by dividing copies of MHC class II by copies of 18S rRNA. Results Phosphorylated CIITA appears predominantly in the nucleus During the course of previous studies of CIITA, we observed that the protein migrates more slowly on SDS-polyacrylamide gels than would be expected for an 1130-aa protein. FgCIITA migrates as doublet through denaturing gels, with protein sizes of 149 and 145 kDa as indicated on immunoblots (Fig. 1A, lane 1). We reasoned that one of these bands may be indicative of a phosphorylated form of CIITA. When preincubated with a broad-acting protein phosphatase capable of dephosphorylating serine, threonine, and tyrosine residues, the upper 149-kDa band of CIITA completely disappears (compare lanes 1 and 2). Likewise, CIITA translated in vitro in the absence of any kinase also migrates only as the lower band, 145-kDa protein (Fig. 1A, lane 3, and Fig. 1B, lane 7). Therefore, it appears that the upper, 149-kDa band represents the phosphorylated form of CIITA, whereas the lower, 145kDa band represents the less phosphorylated form. It should be 378 PHOSPHORYLATION AND INHIBITION OF NUCLEAR CIITA FIGURE 1. CIITA present in the nucleus is in a predominantly phosphorylated form. A, Immunoblot of extracts from COS cells transfected with wildtype CIITA (lanes 1 and 2), CIITA fused to an NLS at its C terminus (CIITANLS), which shows strong nuclear localization, or CIITA containing a deletion of the five amino acids comprising NLS2 (CIITA⌬955–959), which is localized to the cytoplasm. Extracts from cells transfected with wildtype CIITA were initially treated with protein phosphatase (lane 2). In vitro transcribed and translated wild-type CIITA (i.v. CIITA) is shown in lane 3. All CIITA constructs contained an N-terminal FLAG epitope and were detected using an antiFLAG Ab. B, Phosphatase treatment of CIITA eliminates the larger form of the protein. COS cells were transfected with the indicated constructs, and extracts were incubated in the absence (–) or the presence (⫹) of protein phosphatase before immunoblot analysis as in A. C, Immunofluorescence of wild-type (CIITA), nuclear (CIITANLS), and cytoplasmic (CIITAD955–959) forms of CIITA. Expression plasmids were transfected into COS cells. Fluorescence indicates the subcellular localization of the protein. D, Immunoblot of subcellular fractionations of COS cells transfected with wild-type CIITA. Cytoplasmic and nuclear fractions were separated by electrophoresis, and the doublet pattern of CIITA was detected with an anti-FLAG Ab. The integrity of the extracts was confirmed by immunoblotting for the NF TFIIB in both fractions. Commercially prepared, untransfected, HeLa whole-cell extract was used as a control for TFIIB protein. noted that despite treatment with the phosphatase or in vitro translation, the lower band does not decrease in size, suggesting that this is the minimal molecular mass of unphosphorylated, fulllength CIITA (Fig. 1B). We have previously observed that CIITA is present in both the cytoplasm and the nucleus and that mutating the CIITA gene can alter this differential subcellular localization (19, 20). Addition of a C-terminal NLS to CIITA (CIITANLS) increases the presence of CIITA in the nucleus, whereas deletion of a five-amino acid NLS motif (residues 955–959) missing in the CIITA gene of bare lymphocyte syndrome patients (CIITA⌬955–959) causes retention of the protein in the cytoplasm (Fig. 1C). Upon immunoblot examination, we found that cells transfected with CIITANLS showed an increase in the presence of phospho-CIITA relative to wild-type (Fig. 1A, lane 4), whereas cells transfected with CIITA⌬955–959 exhibited a marked decrease in the amount of phosphorylated protein present in extracts (lane 5). These results were confirmed by treatment of each of these CIITA constructs with phosphatase, which eliminated only the upper band while leaving untouched the lower band, representing the smaller form of CIITA (Fig. 1B). This suggests that CIITA present in the nucleus is predominantly phosphorylated, whereas CIITA remaining in the cytoplasm is only minimally phosphorylated. To confirm that CIITA is differentially phosphorylated depending on its subcellular localization, we prepared nuclear and cytoplasmic extracts from cells transfected with wild-type CIITA. Cytoplasmic fractions of transfected cells contain almost exclu- sively the 145-kDa, and thus unphosphorylated, form of CIITA, whereas both unphosphorylated and the more abundant 149-kDa phosphorylated CIITA are present in nuclear extracts (Fig. 1D). The integrity of the extracts was confirmed by blotting for the nuclear basal transcription factor TFIIB and comparing to the known size of TFIIB derived from commercially prepared HeLa cell extracts. Identification of potential sites of phosphorylation on CIITA To identify sites of phosphorylation within CIITA, various N-terminal deletions were made and assayed for loss of the upper, phosphorylated form of CIITA in extracts of transfected cells (Fig. 2). Deletion of aa 1–335 resulted in a single, unphosphorylated protein band detected on immunoblots (Fig. 2A), suggesting that phosphorylation occurs in aa 1–335. An internal deletion of aa 132–301 also resulted in a single, unphosphorylated CIITA band, further delineating the region containing possible sites of phosphorylation. Deletion of increasing amounts of the C terminus revealed that CIITA constructs of residues 1–186, 1–224, and 1–260 all remained as single unphosphorylated forms (Fig. 2B). In contrast, CIITA 1–306 appears as a double band, and loss of the upper, phosphorylated band is evident after treatment with phosphatase. Further mapping of potential phosphorylation sites by immunoblots of CIITA deletion mutants suggested that phosphorylation leading to the appearance of a CIITA doublet occurs between residues 282 and 293 (Fig. 2C). Phosphoamino acid peptide analysis The Journal of Immunology 379 tively), whereas mutation of serine 288 alone (S288A) resulted in a relative decrease in the upper, phosphorylated form of CIITA (lane 3). Combined S286/288A or S288/293A mutations have the most pronounced effect on formation of the CIITA doublet, resulting in a marked decrease in the presence of phosphorylated CIITA (lanes 5 and 7). Interestingly, the S286/293A double mutant showed increased levels of phosphorylation. Potentially, this mutant causes an alteration in the CIITA structure that permits phosphorylation of other residues in more distant regions of the protein, although this possibility remains to be investigated. Mutation of all three serine residues together resulted in a relatively unstable CIITA protein expressed at a lower efficiency. Therefore, we focused on single and double serine mutants in the course of examining their effects on phosphorylation and localization. Mutation of serine residues induces nuclear localization of CIITA FIGURE 2. Deletion mapping identifies phosphorylated serine residues in CIITA. Immunoblot analyses of extracts from COS cells transfected with the indicated FLAG-tagged wild-type or mutant CIITA genes. A, The doublet CIITA bands are present in wild-type CIITA-transfected cells, but absent in extracts from cells transfected with CIITA missing the N-terminal 335 aa (⌬1–335) or CIITA containing an internal deletion of residues 132– 301 (⌬132–301). B, Detailed mapping of the region of CIITA between residues 186 and 306. Cells were transfected with C-terminal deletion mutants of CIITA, such that the mutant CIITA proteins contained only the indicated amino acids. Ten micrograms from each extract was incubated with protein phosphatase and compared with untreated extracts after SDSPAGE separation and immunoblot. Note the loss of the upper band after phosphatase treatment of extracts from cells transfected with CIITA1–306. C, Fine mapping of the region between aa 260 and 306. Loss of the upper band detected with the anti-FLAG Ab in CIITA1–293 coincides with deletion of the adjacent 11 aa generating CIITA1–282. D, Banding patterns from extracts of cells transfected with CIITA constructs mutated at the three serine residues between aa 282 and 293. Serine-to-alanine mutations were made individually at serine 286, 288, or 293 (lanes 2– 4) and in combinations in which two serine residues were mutated together (lanes 5–7). The ratio of the upper, phosphorylated band to the lower, unphosphorylated band in each lane is indicated at the bottom. The SD is indicated from three independent experiments. E, Amino acid sequence of the region between aa 282 and 312 of CIITA. Potential phosphorylated residues identified above are boxed. suggests that CIITA is phosphorylated exclusively on serine residues (32), and within this region of CIITA are three serine residues at aa 286, 288, and 293 (Fig. 2E). We analyzed these particular residues by mutating each of these sites alone or in combination with one another (Fig. 2D). Mutation to alanine of serine 286 (S286A) and serine 293 (S293A) did not result in a dramatic change in the proportion of the two forms (lanes 2 and 4, respec- As immunoblot analyses indicate that the nuclear form of CIITA appears to be heavily phosphorylated, whereas the less phosphorylated CIITA form resides in the cytoplasm, we determined whether mutation of the serine residues alters CIITA localization. COS cells were transfected with wild-type CIITA or CIITA mutated at serines 286, 288, and 293 (S286A, S288A, and S293A, respectively). Immunofluorescence of these transfected cells showed that wild-type CIITA is normally present in both the cytoplasm and the nucleus (Fig. 3), agreeing with previous studies (19, 20). As single-point mutations showed a slight increase in the nuclear concentration of CIITA, double-point mutations (S286/ 288A, S288/293A, and S286/293A) were also examined for their effects on CIITA localization. Each of the CIITA double mutants showed an increase in nuclear concentration of CIITA, with the mutants that include the serine-to-alanine mutation at residue 288 (S286/288A and S288/293A) showing a significant increase in nuclear concentration. Thus, whereas mutation of the serine residues alone only mildly increases CIITA in the nucleus, double mutations, especially mutation of serine 288 in conjunction with a mutation at either of the other two serine sites (S286 or S293), are sufficient to enhance CIITA’s presence in the nucleus. If serine phosphorylation is responsible for translocation of CIITA from the cytoplasm into the nucleus, then it would be expected for the mutants to demonstrate increased cytoplasmic localization. However, the opposite was observed, indicating that phosphorylation of these sites is not required for nuclear import. Instead, phosphorylation at these residues may act as a subsequent signal to target CIITA for nuclear export and/or proteasomal degradation and thereby alter CIITA function. Effect of phosphorylation on CIITA self-association or interaction with other proteins If phosphorylation is necessary to modulate nuclear CIITA function, we explored the possibility that phosphorylation specifically alters the critical interactions of CIITA with itself or other factors. CIITA undergoes self-association, mediated by several domains spread throughout the N terminus, the nucleotide binding domain and leucine-rich region (21, 33, 34, 40). In addition, nuclear CIITA interacts with transcription factors such as RFX5 and NF-Y in trimeric complexes at the promoters of MHC class II target genes to activate transcription. To determine whether the phosphorylated or unphosphorylated form of CIITA self-associates or interacts with transcription factors to form the MHC class II enhanceosome, we performed coimmunoprecipitations of CIITA from extracts of transfected cells. Cells were transfected with FLAG-tagged CIITA (FgCIITA) and CIITA, RFX5, or NF-YB tagged with the Myc epitope. Extracts were immunoprecipitated with anti-FLAG or anti- 380 FIGURE 3. Double-point mutations that include serine 288 increase the nuclear localization of CIITA. COS cells were transfected with the indicated FLAG-tagged constructs, and immunofluorescence was performed 24 h post-transfection using an anti-FLAG Ab to detect the subcellular localization of CIITA. All cells were treated with actinomycin D for 3 h before harvest to ensure that any cytoplasmic protein was not simply the result of new synthesis. Samples were compared with the relatively even cellular distribution of wild-type CIITA. Myc Abs, and complexes were detected by immunoblot with the anti-FLAG Ab (Fig. 4A). In each set, the control immunoprecipitation with anti-FLAG detected both phosphorylated and unphosphorylated CIITA, indicating the presence of both species within the cell. Immunoprecipitations with anti-Myc Ab, followed by immunoblot with anti-FLAG to detect CIITA-associated CIITA or RFX5, predominantly detected the higher molecular mass phosphorylated form of CIITA, suggesting that the phosphorylated form may preferentially associate with CIITA and RFX5 (Fig. 4A, lanes 1– 6). A similar experiment performed with NF-Y also detected the higher molecular mass phosphorylated form of CIITA in association with NF-Y. The preferential association of CIITA and RFX5 with a phosphorylated form of CIITA is in agreement with previous publications (33, 34). As CIITA preferentially immunoprecipitated the phosphorylated form of itself, self-association of CIITA appears to be dependent on the state of protein phosphorylation. To confirm this result, extracts from cells transfected with FgCIITA and MycCIITA were incubated with the protein phosphatase before immunoprecipitation (Fig. 4B). In the absence of protein phosphatase, the phosphorylated band of CIITA was preferentially coprecipitated, as observed in Fig. 4A. However, despite treatment with the phosphatase, ex- PHOSPHORYLATION AND INHIBITION OF NUCLEAR CIITA FIGURE 4. Interaction of CIITA with itself or other proteins is not dependent on phosphorylation of CIITA. A, Extracts from cells transfected with FLAG-tagged CIITA (FgCIITA) and cotransfected with Myc-tagged CIITA, RFX5, or NF-YB were subjected to immunoprecipitation with antiFLAG or anti-Myc Abs or were incubated with anti-Ig beads alone (⫺). FLAG-tagged immunoprecipitated proteins were detected by immunoblotting with the anti-FLAG Ab. Note the presence of both phosphorylated and unphosphorylated FgCIITA coimmunoprecipitating with the MycCIITA, MycRFX5, and MycNF-YB. B, Dephosphorylation does not eliminate selfassociation of CIITA. Extracts from FgCIITA- and MycCIITA-transfected cells were subjected to phosphatase treatment, then immunoprecipitated as described in A. tracts immunoprecipitated with anti-Myc Ab were able to coimmunoprecipitate FLAG-tagged CIITA, indicating that FgCIITA and MycCIITA remained associated despite treatment with phosphatase. Taken together, the data indicate that in the presence of both forms of the protein, there was a preferential association between the phosphorylated forms. However, removal of the phosphate groups did not disrupt homodimerization. There can be at least two interpretations: one is that dephosphorylated CIITA is the less preferred form for self-association, but in the absence of phosphorylated CIITA, its self-associative property becomes more evident. Alternatively, it is possible that phosphorylation is required for self-association, but not for the maintenance of self-association. Hence, once CIITA self-associates in cells, the removal of phosphates has no effect on self-associated dimers or multimers. Double mutation of serine residues has a small effect on CIITA trans-activation of an artificial promoter-reporter system We next examined the effect of phosphorylation on CIITA-mediated gene activation. COS and HeLa cells were cotransfected with wild-type CIITA or the serine mutants along with a luciferase reporter under the control of the MHC class II HLA-DR promoter. CIITA activated expression from the DR promoter by 25- to 40fold in both cell lines. Single-point mutants have minimal impact on altering activation of the MHC class II promoter (Fig. 5A). Double-serine mutants, S286/288A and S288/293A, both modestly The Journal of Immunology 381 FIGURE 6. CIITA phospho-mutants increase the expression of endogenous MHC class II genes. Real-time PCR analysis was performed to measure endogenous mRNA levels of class II in HeLa cells transfected with CIITA phosphorylation mutants. Eighteen hours after cell culture, HeLa cells were transfected with 500 ng of pCDNA3 (control), wild-type CIITA, or CIITA double mutants, and mRNA was isolated 24 h post-transfection. Samples were normalized to the number of 18S rRNA copies. The data shown are representative of three experiments. with wild-type CIITA. Therefore, loss of phosphorylation within the region of S286-S293 of CIITA leads to increased activation of endogenous MHC class II genes, suggesting that phosphorylation of CIITA at the indicated amino acids served to down-regulate the normal protein activity. FIGURE 5. Mutation of serine 288 in double-serine mutants enhances the trans-activation ability of CIITA. Serine-to-alanine mutations at residues 286, 288, and 293 were assayed singly (A) or in each double combination (B). COS (f) or HeLa cells (^) were transfected with wild-type CIITA or the indicated CIITA mutants along with the luciferase reporter under control of the class II DR promoter. One hundred percent activity in each cell type was defined as the level of DR-luciferase activation by wildtype CIITA. All other values were plotted as percentages of this activity. All experiments were performed in triplicate and normalized to protein concentration. enhanced activation by 30 – 60% more than that seen for wild-type CIITA, whereas the S286/293A mutant showed activity levels comparable to those of wild-type CIITA (Fig. 5B). These levels are too modest, particularly for a promoter-reporter system, to permit us to reach a conclusion about the effects of these serine mutations on CIITA activity. Double mutation of serine residues significantly enhanced CIITA trans-activation of an endogenous MHC class II gene The transfected DR-luciferase reporter construct used above represents an artificial system without the benefit of fully assembled chromatin structures at the tested promoter. To thoroughly assess the impact of phosphorylation on CIITA activity, it was important to establish the effects of CIITA phosphorylation on regulation of the expression of endogenous MHC class II genes. Cells transfected with wild-type CIITA or double-point mutants were examined for levels of endogenous MHC class II gene expression, as measured by quantitative real-time PCR analysis (Fig. 6). The expression of MHC class II genes induced by double mutants of CIITA was compared with that induced by wild-type CIITA expression. Surprisingly, cells transfected with S288/293A and S286/ 293A mutants consistently showed a 3- to 5-fold increase in levels of MHC class II gene expression over that seen in cells transfected Discussion CIITA has been well characterized as a transcriptional activator operating at the promoter of MHC class II target genes. Recent evidence suggests that CIITA is present in the cytoplasm as well (18, 19) due to export of the protein from the nucleus (20, 27). The question arises of how this dual presence is controlled and what modifications of CIITA may regulate its localization and activity. One mechanism commonly used by cells to control protein function is that of phosphorylation. Numerous kinase pathways are activated within the cell after the stimulation of cell surface receptors with a variety of cytokines or other factors. For example, the expression of MHC class II genes is stimulated by IFN-␥ via activation of the Janus kinases through Stat1 and IFN regulatory factor-1 activation of CIITA expression (1, 41, 42). The function or localization of a variety of other proteins has been shown to be modulated by phosphorylation (43). In this paper we have attempted to further define the roles of specific serine residues as targets of phosphorylation and as residues important for modulating CIITA localization and activity. Subcellular fractionations and immunoblot assays indicated that nuclear CIITA is predominantly phosphorylated, although a smaller amount of unphosphorylated CIITA is still evident in nuclear fractions. In contrast, CIITA in the cytoplasm is primarily unphosphorylated, suggesting that either phosphorylated CIITA is immediately transported from the cytoplasm into the nucleus or that after import into the nucleus, unphosphorylated CIITA is targeted by a nuclear kinase. In vitro phosphopeptide-mapping studies have indicated multiple sites of serine phosphorylation on CIITA (32). Through analysis of deletion mutants, we have narrowed the region down to three serine residues, aa 286, 288, and 293, which serve as primary targets of phosphorylation. Deletion of a region containing these residues results in a form of CIITA that is unaffected by treatment with the protein phosphatase. Site-specific mutation of any two of these sites caused varying 382 ‘degrees of altered phosphorylation; however, some phosphorylated CIITA remained. Mutation of all three resulted in very low protein expression, probably attributed to protein instability, such that the results are inconclusive (not shown). Although these experiments indicate that these three serine residues probably encompass major phosphorylation sites, recent studies have suggested additional regions between the proline-serine-threonine and GBD of CIITA as targets of PKA-mediated phosphorylation (34). Another paper showed that the region 253–321 contains the phosphorylation sites, which agrees with our mapping of serines 286, 288, and 293 as the most probable sites, although additional serines within that region may contribute to global CIITA phosphorylation (33). The possible roles of phosphorylation of CIITA include regulating nuclear transport, protein-protein interactions, or gene activation. The phosphorylated form is more prominent in the nucleus, whereas the nonphosphorylated form is more prominent in the cytoplasm. This was shown in a number of experiments, including cell fractionation and the test of mutants that primarily localize to the cytoplasm or nucleus. This indicates that the coupling of phosphorylation and nuclear import may occur very rapidly, such that little of the phosphorylated cytoplasmic form is ever detected, or that CIITA is primarily phosphorylated in the nucleus. We favor the latter explanation, as mutations of these sites did not block nuclear translocation and actually increased nuclear localization of the protein. This increase in the nuclear concentration of the phosho-mutant form of CIITA suggests that phosphorylation within the region of aa 286 –293 occurs within the nucleus and serves to decrease nuclear CIITA in one of two ways: it may facilitate export of CIITA back to the cytoplasm, or it may induce degradation of the phosphorylated CIITA protein in the nucleus. Therefore, we suggest that the ability of CIITA to enter the nucleus is independent of the state of phosphorylation of residues 286 – 293. Subsequent phosphorylation of serines within this region affects nuclear CIITA by inducing a decrease in the total nuclear concentration of the protein. With regard to the roles of phosphorylation and protein interaction, both underphosphorylated and phosphorylated CIITA can interact with RFX5 and NF-YB, although the phosphorylated form interacts better with RFX5. Data for NF-YB show a similar trend, but the evidence was less conclusive. Self-association of CIITA also appears to prefer the phosphorylated form, but dephosphorylation by protein phosphatase does not eliminate CIITA self-interactions. These observations indicate either that dephosphorylation does not disrupt preformed CIITA dimers/multimers, or that in the absence of phosphorylated CIITA, which serves as a superior partner, dephosphorylated CIITA can undergo self-association. The enhanced association of phosphorylated CIITA with itself and with RFX5 led us to initially hypothesize that phosphorylation of CIITA at S286, S288, and S293 is probably a positive regulatory step, similar to that proposed by others (33, 34). Our initial trials with mutants of S286/288A and S288/293A showed a small increase in trans-activation ability in promoter reporter assays; however, the changes were very slight, such that conclusions could not be drawn. Surprisingly, further analysis of the effects of CIITA double mutations on endogenous MHC class II gene expression by real-time PCR indicated that double mutants that included serine 293 showed significantly enhanced CIITA activity. The difference in MHC class II gene expression levels between the promoterreporter luciferase assay and the real-time PCR experiments may be due to the fact that only the latter measured CIITA-mediated gene expression from intact chromatin and was therefore a more physiological assessment of promoter activation. Thus, phosphorylation of individual serines 288 and 293 as well as phosphoryla- PHOSPHORYLATION AND INHIBITION OF NUCLEAR CIITA tion within the region of residues 286 –293 may play a role in down-regulating CIITA activity. The increased trans-activation ability of these CIITA phospho-mutants may be directly related to their increased concentration within the nucleus, as is apparent in immunofluorescence assays. Therefore, the role of phosphorylation at these residues may be to decrease the nuclear concentration of CIITA. As suggested previously, this may be achieved by enhancing protein degradation or nuclear export. A direct consequence of this decrease in nuclear CIITA is the down-regulation of CIITA-mediated gene expression, as evidenced by the increased levels of endogenous MHC class II message seen in the presence of CIITA phosphorylation mutants. There are several explanations to interpret these data in the light of the earlier data. Data presented in this report and in two earlier reports indicate that the phosphorylated form of CIITA enhances self-association and interaction with RFX5 and NF-Y (33, 34). Those studies also implicate a positive effect of kinases and a negative effect of phosphatases on CIITA function. In contrast, we demonstrate that specific mutation of serines 288 and 293 decreases the amount of the phosphorylated form of CIITA and increases CIITA-mediated endogenous gene expression. It is important to note that Tosi et al. (33) did not test CIITA mutants that lacked the region of phosphorylation in a trans-activation assay, but inferred the importance of phosphorylation as a positive regulatory step by the use of phosphatase or phosphatase inhibitors. In experiments in which they transfected recombinant CIITA into U937 cells, the protein was predominantly nonphosphorylated, and little MHC class II was detected, unless PMA was added, in which case CIITA was phosphorylated and MHC class II expression was increased. However, CIITA mutants again were not tested (33). Sisk et al. (34) focused on in vitro phosphorylation of CIITA by PKA to demonstrate that maximal CIITA activity requires PKA phosphorylation in the region of CIITA aa residues 325– 408. This constitutes a different issue and concerns PKA-induced phosphorylation, which is known to either enhance or reduce MHC class II depending on the cell type. A specific mutant bearing mutations in all four serine residues within the target region showed decreased activity compared with wild-type CIITA; however, the effect was modest, and only a reporter system was used. Each study used different approaches to identify a role for phosphorylation in regulating CIITA activity. The most likely conclusion is that a complex pattern of phosphorylation by different kinases on different residues of CIITA in response to different stimuli may result in divergent outcomes. Phosphorylation can lead to enhanced CIITA self-association and association with other DNA-binding factors and probably enhanced transcription. In contrast, the specific region of residues 286 –293 has a negative regulatory function. One caveat is that the functional effect of mutating residues 293 in combination with 286 or 288 may be unrelated to phosphorylation and simply due to a change of amino acid side chains. We cannot rule out this possibility at present, although this region clearly contains the predominant phosphorylation sites, as removal of this region reduced the phosphorylated form. The data presented in this paper suggest the following model for the role of phosphorylation of aa S286, S288, and S293 on CIITA activity. Cytoplasmic CIITA is relatively underphosphorylated. Phosphorylation of CIITA probably occurs in the nucleus or concurrently with translocation of the protein into the nucleus, as the phosphorylated form of CIITA is found predominantly associated with nuclear fractions. CIITA may be phosphorylated at sites other than S286, S288, and S293 to cause preferential association with RFX5 and other transcription factors to activate the MHC class II target genes. As a molecular switch, specific phosphorylation of The Journal of Immunology CIITA residues S286, S288, and S293 later causes down-regulation of its trans-activation ability. This may lead to enhanced degradation of CIITA or increased export to reduce the nuclear localization of CIITA and its trans-activation function, both of which can be tested. Furthermore, CIITA with mutated S286, S288, and S293 should not exhibit reduced ability to associate with RFX and other transcription factors and may even exhibit enhanced associative capacity, correlating with its enhanced ability to activate the endogenous MHC class II gene. Various aspects of this model are testable, and future experiments should allow us to better decipher the relevance of these three serine residues as well as other phosphorylated residues in modifying the function of CIITA. Acknowledgments We thank J. MacKeigan and X. Zhu for assistance with plasmid preparation, S. Kratovac for technical assistance, and G. Li for advice on phosphatase assays. References 1. Steimle, V., C. A. Siegrist, A. Mottet, B. Lisowska-Grospierre, and B. Mach. 1994. Regulation of MHC class II expression by interferon-␥ mediated by the transactivator gene CIITA. Science 265:106. 2. Chin, K. C., C. Mao, C. Skinner, J. L. Riley, K. L. Wright, C. S. Moreno, G. R. Stark, J. M. Boss, and J. P. Ting. 1994. Molecular analysis of G1B and G3A IFN␥ mutants reveals that defects in CIITA or RFX result in defective class II MHC and Ii gene induction. Immunity 1:687. 3. Chang, C. H., and R. A. Flavell. 1995. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J. Exp. Med. 181:765. 4. Harton, J. A., and J. P. Ting. 2000. Class II transactivator: mastering the art of major histocompatibility complex expression. Mol. Cell. Biol. 20:6185. 5. Taxman, D. J., D. E. Cressman, and J. P. Ting. 2000. Identification of class II transcriptional activator-induced genes by representational difference analysis: discoordinate regulation of the DN␣/DO heterodimer. J. Immunol. 165:1410. 6. Nagarajan, U. M., A. Bushey, and J. M. Boss. 2002. Modulation of gene expression by the MHC class II transactivator. J. Immunol. 169:5078. 7. DeSandro, A., U. M. Nagarajan, and J. M. Boss. 1999. The bare lymphocyte syndrome: molecular clues to the transcriptional regulation of major histocompatibility complex class II genes. Am. J. Hum. Genet. 65:279. 8. Reith, W., and B. Mach. 2001. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 19:331. 9. Scholl, T., S. K. Mahanta, and J. L. Strominger. 1997. Specific complex formation between the type II bare lymphocyte syndrome-associated transactivators CIITA and RFX5. Proc. Natl. Acad. Sci. USA 94:6330. 10. Mahanta, S. K., T. Scholl, F. C. Yang, and J. L. Strominger. 1997. Transactivation by CIITA, the type II bare lymphocyte syndrome-associated factor, requires participation of multiple regions of the TATA box binding protein. Proc. Natl. Acad. Sci. USA 94:6324. 11. Fontes, J. D., N. Jabrane-Ferrat, C. R. Toth, and B. M. Peterlin. 1996. Binding and cooperative interactions between two B cell-specific transcriptional coactivators. J. Exp. Med. 183:2517. 12. Kretsovali, A., T. Agalioti, C. Spilianakis, E. Tzortzakaki, M. Merika, and J. Papamatheakis. 1998. Involvement of CREB binding protein in expression of major histocompatibility complex class II genes via interaction with the class II transactivator. Mol. Cell. Biol. 18:6777. 13. Fontes, J. D., B. Jiang, and B. M. Peterlin. 1997. The class II trans-activator CIITA interacts with the TBP-associated factor TAFII32. Nucleic Acids Res. 25:2522. 14. Zhu, X. S., M. W. Linhoff, G. Li, K. C. Chin, S. N. Maity, and J. P. Ting. 2000. Transcriptional scaffold: CIITA interacts with NF-Y, RFX, and CREB to cause stereospecific regulation of the class II major histocompatibility complex promoter. Mol. Cell. Biol. 20:6051. 15. Zhou, H., and L. H. Glimcher. 1995. Human MHC class II gene transcription directed by the carboxyl terminus of CIITA, one of the defective genes in type II MHC combined immune deficiency. Immunity 2:545. 16. Riley, J. L., S. D. Westerheide, J. A. Price, J. A. Brown, and J. M. Boss. 1995. Activation of class II MHC genes requires both the X box region and the class II transactivator (CIITA). Immunity 2:533. 17. Chin, K. C., G. G. Li, and J. P. Ting. 1997. Importance of acidic, proline/serine/ threonine-rich, and GTP-binding regions in the major histocompatibility complex class II transactivator: generation of transdominant-negative mutants. Proc. Natl. Acad. Sci. USA 94:2501. 18. Harton, J. A., D. E. Cressman, K. C. Chin, C. J. Der, and J. P. Ting. 1999. GTP binding by class II transactivator: role in nuclear import. Science 285:1402. 383 19. Cressman, D. E., K. C. Chin, D. J. Taxman, and J. P. Ting. 1999. A defect in the nuclear translocation of CIITA causes a form of type II bare lymphocyte syndrome. Immunity 10:163. 20. Cressman, D. E., W. J. O’Connor, S. F. Greer, X. S. Zhu, and J. P. Ting. 2001. Mechanisms of nuclear import and export that control the subcellular localization of class II transactivator. J. Immunol. 167:3626. 21. Kretsovali, A., C. Spilianakis, A. Dimakopoulos, T. Makatounakis, and J. Papamatheakis. 2001. Self-association of class II transactivator correlates with its intracellular localization and transactivation. J. Biol. Chem. 276:32191. 22. Spilianakis, C., J. Papamatheakis, and A. Kretsovali. 2000. Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol. Cell. Biol. 20:8489. 23. Harton, J. A., E. Zika, and J. P. Ting. 2001. The histone acetyltransferase domains of CBP and pCAF are not necessary for cooperativity with CIITA. J. Biol. Chem. 20:20. 24. Greer, S. F., E. Zika, B. Conti, X. S. Zhu, and J. P. Ting. 2003. Enhancement of CIITA transcriptional function by ubiquitin. Nat. Immunol. 5:3091. 25. Zika, E., S. F. Greer, X. S. Zhu, and J. P. Ting. 2003. Histone deacetylase 1/mSin3A disrupts ␥ interferon-induced CIITA function and major histocompatibility complex class II enhanceosome formation. Mol. Cell. Biol. 23:3091. 26. Camacho-Carvajal, M. M., S. Klingler, F. Schnappauf, S. B. Hake, and V. Steimle. 2004. Importance of class II transactivator leucine-rich repeats for dominant-negative function and nucleo-cytoplasmic transport. Int. Immunol. 16:65. 27. Raval, A., J. D. Weissman, T. K. Howcroft, and D. S. Singer. 2003. The GTPbinding domain of class II transactivator regulates its nuclear export. J. Immunol. 170:922. 28. Kehlenbach, R. H., and L. Gerace. 2000. Phosphorylation of the nuclear transport machinery down-regulates nuclear protein import in vitro. J. Biol. Chem. 275:17848. 29. Hagting, A., M. Jackman, K. Simpson, and J. Pines. 1999. Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr. Biol. 9:680. 30. Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930. 31. Komeili, A., and E. K. O’Shea. 1999. Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 284:977. 32. Li, G., J. A. Harton, X. Zhu, and J. P. Ting. 2001. Downregulation of CIITA function by protein kinase A (PKA)-mediated phosphorylation: mechanism of prostaglandin E, cyclic AMP, and PKA inhibition of class II major histocompatibility complex expression in monocytic lines. Mol. Cell. Biol. 21:4626. 33. Tosi, G., N. Jabrane-Ferrat, and B. M. Peterlin. 2002. Phosphorylation of CIITA directs its oligomerization, accumulation and increased activity on MHCII promoters. EMBO J. 21:5467. 34. Sisk, T. J., K. Nickerson, R. P. Kwok, and C. H. Chang. 2003. Phosphorylation of class II transactivator regulates its interaction ability and transactivation function. Int. Immunol. 15:1195. 35. Giroux, M., M. Schmidt, and A. Descoteaux. 2003. IFN-␥-induced MHC class II expression: transactivation of class II transactivator promoter IV by IFN regulatory factor-1 is regulated by protein kinase C-␣. J. Immunol. 171:4187. 36. Piskurich, J. F., Y. Wang, M. W. Linhoff, L. C. White, and J. P. Ting. 1998. Identification of distinct regions of 5⬘ flanking DNA that mediate constitutive, IFN-␥, STAT1, and TGF--regulated expression of the class II transactivator gene. J. Immunol. 160:233. 37. Basta, P. V., P. A. Sherman, and J. P. Ting. 1988. Detailed delineation of an interferon-␥-responsive element important in human HLA-DRA gene expression in a glioblastoma multiform line. Proc. Natl. Acad. Sci. USA 85:8618. 38. Cressman, D. E., L. E. Greenbaum, R. A. DeAngelis, G. Ciliberto, E. E. Furth, V. Poli, and R. Taub. 1996. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379. 39. Wong, A. W., W. J. Brickey, D. J. Taxman, H. W. van Deventer, W. Reed, J. X. Gao, P. Zheng, Y. Liu, P. Li, J. S. Blum, et al. 2003. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions; parathyroid hormone-related protein treatment of pregnant rats delays the increase in connexin 43 and oxytocin receptor expression in the myometrium: regulation and specificity of MHC2TA promoter usage in human primary T lymphocytes and cell line. Nat. Immunol. 4:891. 40. Linhoff, M. W., J. A. Harton, D. E. Cressman, B. K. Martin, and J. P. Ting. 2001. Two distinct domains within CIITA mediate self-association: involvement of the GTP-binding and leucine-rich repeat domains. Mol. Cell. Biol. 21:3001. 41. Muhlethaler-Mottet, A., W. Di Berardino, L. A. Otten, and B. Mach. 1998. Activation of the MHC class II transactivator CIITA by interferon-␥ requires cooperative interaction between Stat1 and USF-1. Immunity 8:157. 42. Piskurich, J. F., M. W. Linhoff, Y. Wang, and J. P. Ting. 1999. Two distinct ␥ interferon-inducible promoters of the major histocompatibility complex class II transactivator gene are differentially regulated by STAT1, interferon regulatory factor 1, and transforming growth factor . Mol. Cell. Biol. 19:431. 43. Jans, D. A., and S. Hubner. 1996. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol. Rev. 76:651.