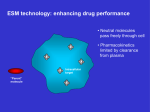
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 5
Ancestral sequence reconstruction wikipedia , lookup
Gene expression wikipedia , lookup
Expression vector wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Interactome wikipedia , lookup
Protein purification wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Structural alignment wikipedia , lookup
SUPERSECONDARY STRUCTURE, DOMAINS AND TERTIARY STRUCTURE Levels of protein structure organization Between secondary and tertiary structure • Supersecondary structure: arrangement of elements of same or different secondary structure into motifs; a motif is usually not stable by itself. • Domains: A domain is an independent unit, usually stable by itself; it can comprise the whole protein or a part of the protein. The Ramachandran map Conformations of a terminally-blocked amino-acid residue E Zimmerman, Pottle, Nemethy, Scheraga, Macromolecules, 10, 1-9 (1977) C7eq C7ax Secondary Structure Preferences • • • • • • • • • Alanine Glutamic Acid Glutamine Leucine Lysine Methionine Phenylalanine helix 1.42 1.39 1.11 1.41 1.14 1.45 1.13 strand 0.83 1.17 1.10 1.30 0.74 1.05 1.38 turn 0.66 0.74 0.98 0.59 1.01 0.60 0.60 Subset of helix-lovers. If we forget alanine (I don’t understand that things affair with the helix at all), they share the presence of a (hydrophobic) C-b, Cg and C-d (S-d in Met). These hydrophobic atoms pack on top of each other in the helix. That creates a hydrophobic effect. Secondary Structure Preferences • • • • • • • • Isoleucine Leucine Phenylalanine Threonine Tryptophan Tyrosine Valine helix 1.08 1.41 1.13 0.83 1.08 0.69 1.06 strand 1.60 1.30 1.38 1.19 1.37 1.47 1.70 turn 0.47 0.59 0.60 0.96 0.96 1.14 0.50 • Subset of strand-lovers. These residues either have in common their bbranched nature (Ile, Thr, Val) or their large and hydrophobic character (rest). Secondary Structure Preferences • • • • • • • helix Aspartic Acid 1.01 Asparagine 0.67 Glycine 0.57 Proline 0.57 Serine 0.77 strand 0.54 0.89 0.75 0.55 0.75 turn 1.46 1.56 1.56 1.52 1.43 Subset of turn-lovers. Glycine is special because it is so flexible, so it can easily make the sharp turns and bends needed in a b-turn. Proline is special because it is so rigid; you could say that it is pre-bend for the b-turn. Aspartic acid, asparagine, and serine have in common that they have short side chains that can form hydrogen bonds with the own backbone. These hydrogen bonds compensate the energy loss caused by bending the chain into a b-turn. Dominant b-turns Idealized hydrogen-bonded helical structures: 310-helix (left), a-helix (middle), p-helix (right) Proline helices (without H-bonds) Polyproline helices I, II, and III (PI, PII, and PIII): contain proline and glycine residues and are left-handed. PII is the building block of collagen; has also been postulated as the conformation of polypeptide chains at initial folding stages. f and y angles of regular and polyproline helices Structure F Y w a-helix -57 -47 180 +3.6 1.5 310-helix -49 -26 180 +3.0 2.0 p-helix -57 -70 180 +4.4 1.15 Polyproline I -83 +158 0 +3.33 1.9 Polyproline II -78 +149 180 -3.0 3.12 Polyproline III -80 +150 180 +3.0 3.1 residues/turn turns/residue Length of a-helices in proteins 10-17 amino acids on average (3-5 turns); however much longer helices occur in muscle proteins (myosin, actin) Antiparallel sheet (L6-7) The side chains have alternating arrangement; usually hydrophobic on one and hydrophilic on the opposite site resulting in a bilayer 2TRX.PDB Parallel sheet (L6-7) The amino acid R groups face up & down from a beta sheet 2TRX.PDB Structure F Antiparallel b Y w Residues/turn Distance along axis/turn -139 +135 -178 2.0 3.4 Parallel b -119 +113 180 2.0 3.2 a-helix -57 -47 180 3.6 1.5 310-helix -49 -26 180 3.0 2.0 p-helix -57 -70 180 4.4 1.15 Polyproline I -83 +158 0 3.33 1.9 Polyproline II -78 +149 180 3.0 3.12 Polyproline III -80 +150 180 3.0 3.1 A diagram showing the dihedral bond angles for regular polypeptide conformations. Note: omega = 0º is a cis peptide bond and omega = 180º is a trans peptide bond. Schemes for antiparallel (a) and parallel (b) b-sheets b-sheets are pleated b-sheet chirality Because of interactions between the side chains of the neighboring strands, the b-strands have left-handed chirality which results in the right twist of the b-sheets N-end C-end Length of b-sheets in proteins 20 Å (6 aa residues)/strand on average, corresponding to single domain length Usually up to do 6 b-strands (about 25 Å) Usually and odd number of b-strands because of better accommodation of hydrogen bonds in a b-sheet Structural motifs (supersecondary structure) b-hairpin I b-hairpin II b-corner) helix hairpin a-a corner E-F hand helix-turn-helix (HTH) motif three-helix bundle four-helix bundle helix-b-hairpin (zinc finger motif) • bab motif • babab motif (Rossman fold • • • • • • • • • • • b-meander • greek key motif • Swiss, jellyroll or b-sandwich motif • horseshoe motif • b-propellor • b-helix Example of a b-hairpin in bovine pancreatic trypsin inhibitor– BPTI. Example of a protein with two b-hairpins: erabutoxin from whale. Example of a b-meander: aspectrin SH3 domain (1BK2) Example of a b-hairpin: tryptophan zipper (1LE0) Helix Hairpin Alpha alpha corner (L7.24) E-F Hand motif Helix E helix F Troponin C with four EF motifs that bind calcium ions. Because of high content of acidic amino-acid residues with side chains pointing inside the loop, the EF-hand motif constitutes a calciumbinding scaffold in troponin, calmodulin, etc. The Helix-Turn-Helix motif • This motif is characteristic of proteins binding to the major DNA grove. • The proteins containing this motif recongize palindromic DNA sequences. • The second helix is responsible for nucleotide sequence recognition. The Helix-Turn-Helix motif Three-helix bundle (1BDD) Four-helix bundle (3M9H) The a-helix-b-hairpin motif (zinc finger) b-a-b Motif (very important and very frequent) Hydrophobic core between a-helix and b-sheet a/b horseshoe The Greek Key Motif The Greek-key motif as seen in proteins Example of a protein with two Greek key motifs: crystallin C. Four Greek key motifs arranged into two b-barrels. RASMOL - gcrysb.pdb The jellyroll topology Example of a protein with jellyroll topology: Carbohydrate-Binding Module Family 28 from Clostridium josui Cel5A (3ACI) Example of a b-barrel (red fluorescent protein; 3NED) The b-helix Example of a b-propellor motif : Thermostable PQQ-dependent Soluble Aldose Sugar Dehydrogenase (3DAS) Classification of three-dimensional structures of protein Richardson’s classification a – a-helices are only or dominant secondary-structure elements (e.g., ferritin, myoglobin) b – b-sheets are only or dominant elements (e.g., lipocain) a/b – contain strongly interacting helices and sheets a+b structures – contain weakly interacting or separated helices and sheets SCOP classification Structural Classification Of Proteins This is a hierarchical classification scheme with the following 4 levels: 1. Families – one family is comprised by proteins related structurally, evolutionally, and functionally. 2. Superfamoilies – A superfamily is comprised by families of substantially related by structure and function. 3. Folds – Superfamilies with common topology of the main portion of the chain. 4. Classes - Groups of folds characterized by secondary structure: a (mainly a-helices), b (mainly b-sheets), a/b (a-helices and bsheets strongly interacting), a+b (a-helices and b-weakly interacting or not interacting), multidomain proteins (nonhomologous proteins with vert diverse folds). [ http://scop.mrc-lmb.cam.ac.uk/scop/ ] CATH classification (Class (C), Architecture(A), Topology(T), Homologous superfamily (H)) Four hierarchy levels: 1. Class (Level C): according to the content of secondary structure type a, b, a&b (a/b and a+b), weakly or undefined secondary structure. 2. Architecture. (Level A) – Orientation and connection topology between secondary structure elements. 3. Topology. (Level T) – based on fold type. 4. Homoloous superfamilies. (Level H) – high homology indicating a common anscestor: - >30% sequence identity OR - > 20% sequence identiy and 60% structural homology OR - > 60% structural homology and similar domains have similar function. • Class(C) derived from secondary structure content is assigned automatically • Architecture(A) describes the gross orientation of secondary structures, independent of connectivity. • Topology(T) clusters structures according to their topological connections and numbers of secondary structures • Homologous superfamily (H) [ http://www.biochem.ucl.ac.uk/bsm/cath_new/ ] Protein periodic table W. Taylor and M. Hill Layers b-sheets: rectangles and circles; a—helics: filled circles