* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cellular Respiration Lecture Notes
Fatty acid metabolism wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Mitochondrion wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Phosphorylation wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
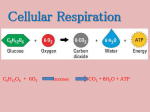
Cellular Respiration Lecture Notes I. Introduction a. ‘Big Picture’ Ideas i. Energy 1. ATP is the only form of energy that the cell can use 2. Used for cell to do work ii. Structure/Function 1. Uses energy from catabolic processes to fuel anabolism b. Important Molecules c. Oxidation/Reduction Reactions i. Loss of electrons for oxidation ii. Gain of electrons for reduction d. Phosphorylation Reactions i. Oxidative phosphorylation 1. 3rd stage of respiration 2. Electrontransport chain accepts electrons from the breakdown of products during the first 2 stages 3. Passes electrons from one molecule to another 4. electrons combined with hydrogen ions 5. molecular oxygen to form water 6. energy released at each step of the chain is stored in mitochondria to make ATP ii. Substrate level phosphorylation 1. Forms smaller amount of ATP 2. Enzyme transfer phosphate group from substrate to an ADP (substrate is organic molecule generated during catabolism of glucose) e. Review Structure of a Mitochondrion II. Glycolysis a. Big Idea i. To harvest chemical energy by oxidating glucose --> 2 pyruvate acids + 2 ATP b. Where Does it occur i. Cytosol (in cytoplasm) c. Molecules In i. Glucose ii. 2 ADP + 2 P (inorganic phosphate) iii. 2 NAD+ d. Molecules Out i. 2 Pyruvate + 2 H2O ii. 2 ATP iii. 2 NADH + 2H+ e. Details to Know i. 1st Step of breaking down sugars into 2 pyruvic + 2 ATP + 2 NADH ii. 10 Steps (1st5 = energy investment, 2nd 5 = energy payoff) iii. Anaerobic= no oxygen required iv. Releases <1/4 energy stored in glucose III. The ‘Intermediate Step’ a. Big Idea i. Conversion of pyruvate to actyl CoA b. Where Does it occur i. Pyruvate enters mitochondion from ctyosol via transport protein c. Molecules In i. CO2, NADH, H+, Acetyl CoA d. Molecules Out i. Pyruvate, NAD+, Coenzyme A e. Details to Know i. Acetyl CoA is necessary to begin Krebs cycle IV. The Krebs Cycle a. Big Idea i. enzymes complete the oxidation of organic fuel to energy with presence of molecular oxygen b. Where Does it occur i. Mitochondrion c. Molecules In i. Acetyl CoA, 3 NAD+, FAD, ADP, + Pi (inorganic phosphate) d. Molecules Out i. 2 CO2, 3 NADH, 3 H+, ATP, FADH2 e. Details to Know f. 8 Steps with a specific enzyme for each step g. Oxidizes organic fuel derived from pyruvate h. 1 ATP released per turn i. Most energy is transferred to NAD+ and FAD j. Energy then goes to electron transport chain to synthesize ATP by oxidative phosphorylation V. The Electron Transport Chain a. Big Idea i. NADH → protiens → drive H+ out of Mitochondrial matrix → let H+ flow through ATP Synthase to make ADP + Pi → ATP b. Where Does it occur i. Mitochondrial matrix, membrane, and intermembrane space c. Molecules In i. NADH & H+ & O2 ii. ADP + Pi d. Molecules Out i. H2O ATP e. Details to Know i. Chemiosmosis – ATP synthesis powered by the flow of H+ back across the membrane (Oxidative phosphorylation) ii. Cytochromes (cyt) – protein electron carriers w/ prosthetic group (heme group) iii. Heme group – 4 organic rings surrounding central iron; similar to hemoglobin but carries electrons, not oxygen iv. ATP Syhtnase – enzyme that makes ATP from ADP & inorganic phosphate 1. Rotor – spins clockwise when H+ flows past it down the conc. Gradient 2. Stator – holds knob stationary, anchored to membrane 3. Rod – also spins, activates catalytic sites in knob 4. Knob – catalytic sites join Pi + ADP → ATP v. Proton-motive force – H+ gradient across membrane VI. Review Respiration Products & Reactants VII. Fermentation a. Big Idea i. Glycolysis + recycling of NADH → NAD+ by transferring electrons to pyruvate or derivatives b. Where Does it occur c. Molecules In i. Alcohol Fermentation 1. 2. Glucose + 2 H+ + 2 NADH ii. Lactic Acid Fermentation 1. 2. Glucose + 2 H+ + 2 NADH d. Molecules Out i. 2 NAD+ + 2 Lactate/2 Ethanol e. Details to Know i. Less efficient that respiration ii. Evolutionarily 1st iii. Anaerobic (no oxygen) VIII. Control of Respiration I. Catabolic Pathways