* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Metabolism of heme
Paracrine signalling wikipedia , lookup
Biochemical cascade wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Lipid signaling wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Peptide synthesis wikipedia , lookup
Wilson's disease wikipedia , lookup
Oligonucleotide synthesis wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
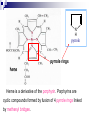
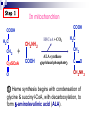
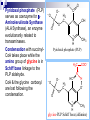
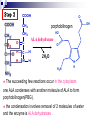
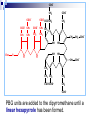
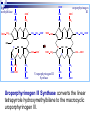
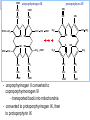
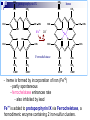
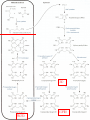
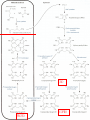
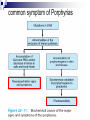
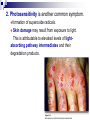
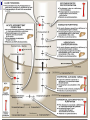
Molecular Biochemistry II Metabolism of heme xiaoli White cell serum platelet Blood cell Read cell hemoglobin globin heme N H pyrrole pyrrole rings heme Heme is a derivative of the porphyrin. Porphyrins are cyclic compounds formed by fusion of 4 pyrrole rings linked by methenyl bridges. Heme is the prosthetic group of hemoglobin, myoglobin, & cytochromes and so on. - most common porphyrin in humans is heme - one ferrous goup in tetrapyrole ring - heme proteins (hemoproteins) are rapidly synthsized and degraded - 6 to 7 g per day hemoglobin turned over - cyclic compounds that bind metals - usually iron - Fe+2 = ferrous - Fe+3 = ferric Synthesis of heme ★ The substrates mainly include succinyl-CoA, glycine, Fe2+ . ★ Heme can be synthesized by almost all the tissues in the body which require hemoproteins. ★major sites of synthes is liver and bone marrow (erythroblasts: reticulocyte, prorubricyte) - cytochrome p450 in liver - hemoglobin in bone marrow - heme production equal to globin synthesis in marrow - variable in liver dependent on heme pool balance Inside RBCs, heme is synthesized in the normoblasts, but not in the matured ones . Inside RBCs, heme is synthesized in the normoblasts, but not in the matured ones . Step 1 In mitochondrion COOH COOH H2C CH 2 + C¡«SCoA O CH 2NH 2 COOH HSCoA + CO2 H2C CH 2 ALA synthase (pyridoxal phosphate) C O CH 2NH 2 ① Heme synthesis begins with condensation of glycine & succinyl-CoA, with decarboxylation, to form d-aminolevulinic acid (ALA). ALA Synthase is the committed step of the heme synthesis pathway, & is usually rate-limiting for the overall pathway. Regulation occurs through control of gene transcription. Heme functions as a feedback inhibitor, repressing transcription of the ALA Synthase gene in most cells. A variant of ALA Synthase expressed only in developing erythrocytes ,is regulated instead by availability of iron in the form of iron-sulfur clusters. H Pyridoxal phosphate (PLP) serves as coenzyme for dAminolevulinate Synthase (ALA Synthase), an enzyme evolutionarily related to transaminases. O O P C H2 C OH O O N CH3 H Pyridoxal phosphate (PLP) Condensation with succinylCoA takes place while the amino group of glycine is in Schiff base linkage to the PLP aldehyde. CoA & the glycine carboxyl are lost following the condensation. O H2C COO N+ HC O O H2 C P O H O O N H CH3 glycine-PLP Schiff base (aldimine) Step 2 COOH O HO CH 2 ALA dehydratase O C H C H H N H O 2H2O H2N ★ The OH porphobilinogen CH 2 N H succeeding few reactions occur in the cytoplasm. one ALA condenses with another molecule of ALA to form porphobilinogen(PBG). ★ the condensation involves removal of 2 molecules of water and the enzyme is ALA dehydratase . the enzyme contains zinc and is very sensitive to lead and other heavy metals. Inhibition of Porphobilinogen Synthase by Pb++ results in elevated blood ALA, as impaired heme synthesis leads to derepression of transcription of the ALA Synthase gene. High ALA is thought to cause some of the neurological effects of lead poisoning, although Pb++ COO COO also may directly affect the nervous system. ALA is toxic to the brain, perhaps due to: • Similar ALA & neurotransmitter GABA (g-aminobutyric acid) structures. • ALA autoxidation generates reactive oxygen species (oxygen radicals). CH2 CH2 CH2 CH2 C O CH2 CH2 NH3+ NH3+ ALA GABA COO COO CH2 H2C NH3+ CH2 CH2 N H Porphobilinogen (PBG) is the first pathway intermediate that includes a pyrrole ring. N H pyrrole Porphobilinogen (PBG) The porphyrin ring is formed by condensation of 4 molecules of porphobilinogen. Porphobilinogen Deaminase catalyzes successive PBG condensations, initiated in each case by elimination of the amino group. COOCOO- CH2 COO- COO-CH COO- Enz S COO- CH2 COO- CH2 CH2 CH2 N H CH2 CH2 2 CH2 CH2 CH2 N H NH HN NH HN CH2 COO- CH2 COOCH2 CH2 CH2 CH2 COO-COO- CH2 COO- PBG units are added to the dipyrromethane until a linear hexapyrrole has been formed. COO- hydroxymethylbilane - OOC COO- uroporphyrinogen III - CH2 COO- CH2 COO CH2 CH2 CH2 CH2 CH2 CH2 NH HN NH HN CH2 COO- - OOC CH2 NH HN NH HN CH2 CH2 COO- CH2 COO- HO C C CH2 C COO- - OOC CH2 C CH2 CH2 CH2 CH2 COO-COO- CH2 COO- Uroporphyrinogen III Synthase CH2 CH2 CH2 CH2 COO- COO- Uroporphyrinogen III Synthase converts the linear tetrapyrrole hydroxymethylbilane to the macrocyclic uroporphyrinogen III. - COO - OOC protoporphyrin IX uroporphyrinogen III CH2 COO- CH2 CH2 CH2 CH CH2 CH2 CH2 - COO CH3 CH CH2 H3C NH HN NH NH HN N - CH2 OOC CH2 COO- N HN H3C CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 COO- COO- COO- COO- - uroporphyrinogen II converted to coproporphyrinonogen III - transported back into mitochondria - converted to protoporphyrinogen IX, then to protoporphyrin IX CH2 protoporphyrin IX CH2 CH CH3 CH CH CH2 H3C NH N N ++ Fe heme CH3 CH CH2 H3C 2H N N + Fe HN N H3C CH3 CH2 CH2 CH2 COO- N H3C Ferrochelatase CH3 CH2 CH2 CH2 CH2 CH2 COO- COO- COO- - heme is formed by incorporation of iron (Fe+2) - partly spontaneous - ferrochelatase enhances rate - also inhibited by lead Fe++ is added to protoporphyrin IX via Ferrocheletase, a homodimeric enzyme containing 2 iron-sulfur clusters. URO COPRO PROTO summary ① major sites of synthes is liver and bone marrow (erythroblasts). Matured red blood cells have no mitochondria, so can’t make heme. ② The substrates mainly include succinyl-CoA, glycine, Fe2+ . ③ first and last 3 reactions take place in mitochondria - others in cytoplasm Regulation of heme synthesis Regulation of heme synthesis 1. ALA synthase Major site of regulation is at the level of ALA synthase. ① It is regulated by repression mechanism. Heme inhibits the synthesis of ALA synthesis by acting as a corepressor. The feedback regulatory effect is a typical example of end-product inhibition. ② ALA synthase is also allosterically inhibited by hematin. When there is excss of free heme without globin chains to bind with, the Fe++ is oxidized to Fe+++ forming hematin. Hematin will inhibit ALA synthase to prevent excessive unwanted production of heme. Hematin will also inhibit the translocation of ALA synthase from the cytoplasm into the mitochondria where its substrate, succinyl CoA is formed. thus heme synthesis is inhibited till there are sufficient globin chains to bind with. ③ Lack of Vit B6 will decrease the synthesis of ALA. Drugs like INH (isonicotinic acid hydrazide) that decrease the availability of pyridoxal phosphate may also affect heme synthesis. 2. Heme synthesis may be inhibited by heavy metals. the steps catalyzed by ALA dehydratase and ferrochelatase are inhibited by lead. 3. erythropoietin, EPO The kidneys also secrete a hormone called erythropoietin. The function of erythropoietin is to stimulate the production of red blood cells. The kidney produces 85~95% of the body's erythropoietin so when the kidney is damaged (kidney disease or failure), not enough erythropoietin is produced to maintain normal red blood cell levels. This leads to anemia. anemia normal URO COPRO PROTO Porphyria Porphyria is a name given to a group of metabolic disorders. These disorders cause the individual to accumulate "porphyrins" or "porphyrin precursors" in their body. which in turn causes an abundance of the porphyrins. In porphyria, the cells do not convert porphyrins to heme in a normal manner. Porphyrias Porphyrias are genetic diseases in which activity of one of the enzymes involved in heme synthesis is decreased (e.g., PBG Synthase, Porphobilinogen Deaminase, etc…). Symptoms vary depending on ★ the enzyme ★ the severity of the deficiency ★ whether heme synthesis is affected primarily in liver or in developing erythrocytes. common symptom of Porphyrias common symptom of Porphyrias 1. Occasional episodes of severe neurological symptoms are associated with some porphyrias. ◆had acute bouts of abdominal pain and mental confusion Permanent nerve damage and even death can result, if not treated promptly. Elevated d-aminolevulinic acid (ALA), arising from derepression of ALA Synthase gene transcription, is considered responsible for the neurological symptoms. 2. Photosensitivity is another common symptom. ◆formation of superoxide radicals. ◆ Skin damage may result from exposure to light. This is attributable to elevated levels of lightabsorbing pathway intermediates and their degradation products. 3. porphyrins build up in the body and are excreted in the urine and stool in excessive amounts. When present in very high levels, they cause the urine to have a spectacular port wine color. normal King George III - Mad King George had acute bouts of abdominal pain and mental confusion - may have been porphyria sufferer - complicated by all the drugs his doctors gave him vampires and wherewolves? - some have put forth that porphyrias misinterpreted in Middle Ages - consider photosensitivity, red blood (even teeth) hypertrichosis Porphyrias can be grouped into erythropoietic porphyria and hepatic porphyria - hepatic can be acute or chronic caused by hereditary or acquired defects in heme synthesis — genetic diseases: the enzymes of heme synthesis — Liver desfunction, lead posioning Acute hepatic porphyrias Each acute hepatic porphyria is a result of a deficiency of one of the enzymes in the heme biosynthesis pathway. These deficiencies result in an accumulation of the precursors of porphyrins in the liver (delta-aminolevulinic acid, ALA and porphobilinogen, PBG) and also, in the case of variagate porphyria and hereditary coproporphyria, an accumulation of porphyrins resulting in cutaneous manifestations. - similar symptoms - acute attacks of gastrointestinal pain, neurologic / psychologic, cardiovascular. When an acute attack is confirmed, urgent treatment with an injection of human hemin and/or perfusion of carbohydrates is required. Management includes the prevention of attacks (by avoiding causal factors) and the protection of skin from the light in cases of cutaneous manifestations. erythropoietic porphyrias - congenital erythropoietic porphyria (uroporphyrinogen III synthase) - erythropoietic protoporphyria (ferrochelatase) symptoms include: - skin rashes and blisters early in childhood - cholestatic liver cirrhosis and progressive liver failure Treatment for Porphyrias - medical support for vomiting and pain - hemin, decreases ALA synthase synthesis - avoidance of sunlight and precipitating drugs, factors porphyria cutanea tarda - a chronic porphyria liver and erythroid tissues deficiencey in uroporphyrinogen decarboxylase often no symptoms until 4th or 5th decade clinical expression determined by many factors: - hepatic iron overload - exposure to sunlight - hepatitis B or C - HIV symptoms include: - cutaneous rashes, blisters - urine that is red to brown in natural light, or pink to red in UV light Acquired Porphyrias - hexochlorobenzene used as a fungicide in Turkey in 1950s - thousands of children ate bread from treated wheat - they acquired porphyria cutanea tarda due to inhibition of uroporphyrinogen decarboxylase - due to hypertrichosis - referred to locally as the “monkey children” Acquired Porphyrias lead poisoning -inhibition of ferrochelatase ALA dehydratase - displaces Zn+2 at enzyme active site children - developmental defects - drop in IQ - hyperactivity - insomnia - many other health problems adults - severe abdominal pain - mental confusion - many other symptoms