* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Citric Acid Cycle
Isotopic labeling wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Butyric acid wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Microbial metabolism wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
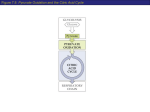
CO2 Biochemistry I Citric Acid Cycle CO2 CO2 Oxidative Decarboxylations and Energy Output Chapter 17 – part 2 Isocitrate Dehydrogenase http://www.wiley.com/college/fob/quiz/quiz16/16-2.html Dr. Ray Key Concept Map For Citric Acid Cycle (TCA) Is TCA cycle an aerobic or anaerobic pathway? Strictly aerobic Cellular Respiration: 1. TCA is first stage, with removal of high energy electrons from carbon fuels. 2. Electrons reduce O2 to generate a proton gradient across Inner Mitochondrial Membrane 3. H+ gradient is used to synthesize ATP. • Oxidative Phosphorylation involves steps (2) and (3). Ref: Lippincott's Illustrated Reviews: Biochemistry, 3rd Ed., Fig 9.10 Step 1: Citrate Synthase Mechanism – Summary • Brings substrates into _____________________________________ close proximity, orienting them, & polarizing bonds • Uses general _________________________ acid-base catalysis • The hydrolysis of the _____________ powers the synthesis of a thioester new molecule from 2 precursors (C-C bond formation) 1. How is the wasteful hydrolysis of acetyl CoA prevented (since this also has a high-energy thioester linkage)? Citrate synthase is well suited to hydrolyze citryl CoA, not acetyl CoA. Induced fit conformational changes prevent undesirable side reactions Oxaloacetate + Acetyl CoA + H2O Citrate + CoASH CO2CH2 HO OH2 C CO2- CH2 CO2- + CoASH Step 2: Aconitase - Isomerization 1) Can a tertiary ROH be oxidized? 2) Can a secondary ROH be oxidized? • The tertiary hydroxyl group is not properly located in the citrate molecule for the oxidative decarboxylations that follow, so it is moved in this step. • Isomerization of citrate to isocitrate: 1. moves 3o alcohol on C3 up to form a 2o alcohol on C2 2. alcohol oxygen is now beta to carboxylate (COO-) attached to C3, which is needed to facilitate oxidative b-decarboxylation in next step Dehydration Rehydration 3o at C3 Isomerization by ACONITASE always moves the central alcohol AWAY from acetyl-CoA side • Aconitase is a metalloenzyme with a [4Fe-4S] iron-sulfur cluster at the active site 2o at C2 The Citric Acid Cycle Problems: 2. What statement is NOT correct about the citrate synthase reaction in the citric acid cycle? A) its products include coenzyme A and citrate B) it forms a tricarboxylic acid C) its substrates include acetyl-CoA and oxaloacetate D) it is coupled to the hydrolysis of ATP E) acetyl CoA is the nucleophile in this reaction 3. The isomerization of citrate to isocitrate: A) is the only unnecessary step of the citric acid cycle. B) protects cells from the toxic effects of carbon monoxide (CO). C) converts a tertiary alcohol, which cannot easily be oxidized, to a secondary alcohol that can be oxidized. D) is a reduction reaction. E) is an oxidation reaction. - Alcohol oxidation will occur in next step Step 3: Isocitrate Dehydrogenase – oxidative decarboxylation 6C Isocitrate 5C a-Ketoglutarate + CO2 The oxidative b-decarboxylation of isocitrate is catalyzed by: Isocitrate Dehydrogenase: C1 C2 C3 C4 b-keto acid C1 b b oxidation a C2 C3 b-decarboxy lation • The rate of formation of a-ketoglutarate is important in determining the overall rate of the citric acid cycle. • This oxidation generates the first high energy electron carrier (NADH) in the cycle (energy extracted via oxidation). The CO2 that is removed began the cycle as part of oxaloacetate (labeled C1, C2, C3, C4 above), not as part of acetyl CoA. Electrostatic Catalysis: b-Decarboxylation Reactions Require an Electron Sink Mechanism of Covalent catalysis via Schiff base (imine) formation: 1. Uncatalyzed Rxn mechanism: • breaking Ca-Cb a b bond requires nearby carbonyl as an electron sink 2. Chemically 1) How can energy catalyzed of anionic enolate mechanism: be lowered? a primary amine that forms a: positively (+) charged imine ELECTRON SINK 2) Why is lower reaction faster than upper reaction? Many decarboxylation reactions occur in the Citric Acid Cycle, and most have an electron sink like a positively charged Schiff Base ! Step 3: Isocitrate Dehydrogenase Loss of CO2 (Mechanism in Chapter 15) b-keto carboxylic acid carbanion + carbon dioxide ketone O H2C b-carbon A --H2C O Oxidative b-decarboxylation ketone + CO2 O B H3C O - O O C O O C O A. Loss of CO2 from a beta-keto acid or equivalent; Only the Ca-Cb bond can by cleaved because the resulting carbanion is resonance stabilized as an enolate; carbanion only stable adjacent (alpha) to a carbonyl • Since carbanions alpha to carbonyl are stabilized by resonance, the b-keto acid can cleave to give this carbanion. Thus only Ca-Cb bonds are cleaved ! B. Reprotonation of the carbanion C by an acid at the active site • When the CO2 is lost, it leaves as a GAS; this reaction is “irreversible” for all practical purposes • Since this reaction is “irreversible,” Nature has had to design another mechanism for adding CO2 to molecules. In almost all cases, addition of CO2 uses the biotin cofactor and requires energy in the form of ATP. Step 3: Isocitrate Dehydrogenase Mechanism of Oxidative b-decarboxylation Know Produces first CO2 and NADH of citric acid cycle via two steps: mechanism 1. Oxidation of 2o alcohol at C2 to a ketone to form oxalosuccinate (betaketo acid), and reduction of NAD+ to NADH 2. Next undergoes b-decarboxylation of carboxylate (beta) to ketone at C2 3. Mn2+ stabilizes enolate O-, which protonates to enol then tautomerizes to more stable ketone forming a-ketoglutarate C5 C4 C3 C2 C1 Earlier isomerization by aconitase always moves the central alcohol AWAY from acetyl-CoA side Came from Acetyl CoA C6 1 2 3 - flow) 1) _________ double bond (encourage e Mn2+ function? 2) _________ intermediate O(-) charge C5 C4 C3 C2 C1 Mechanism of Isocitrate Dehydrogenase oxidative b-decarboxylation (A) (D) (B) (E) Isocitrate + NAD+ a-Ketoglutarate + NADH + CO2 Q: In what order do these steps occur? (C) (F) Lehninger – Principles of Biochemistry, 5th Ed, animations (chapter 16), by Nelson and Cox, 2008 W. H. Freeman & Company Step 4: a-Ketoglutarate Dehydrogenase Oxidative a-decarboxylation 5C a-Ketoglutarate 4C Succinyl-CoA + CO2 • Produces second CO2 and NADH of citric acid cycle, by another oxidative decarboxylation step but this time removing the carboxy group a- to C=O • The substantial energy released upon oxidation of C1 carbon from ketone to a carboxylic acid derivative, is stored as: (1) a reduced NADH (2) a ‘high energy’ thioester (a carboxylic acid derivative) • a-decarboxylation of terminal carboxyl is much more complx than b-decarboxylation, so catalyzed by a multienzyme complex homologous to pyruvate dehydrogenase PDHC (do NOT need to know mechanism) 1) Which step in glycolysis does this most resemble, in terms of energy extraction and recovery? where aldehyde oxidation forms high energy acyl phosphate (1,3-BPG), a molecule that allows substrate level phosphorylation Step 5: Succinyl-CoA Synthetase: Substrate-level Phosphorylation Succinyl-CoA + GDP + Pi Succinate + GTP + CoASH • Succinyl CoA is an energy-rich thioester compound: (1) DG° for the hydrolysis of succinyl CoA is about - 33.5 kJ/mol (2) DGo’ for hydrolysis of ATP to from ADP + Pi is - 30.5 kJ/mol 1) Can succinyl CoA be used for substrate level phosphorylation? • The cleavage of the thioester bond of succinyl CoA is coupled to the phosphorylation of a purine nucleoside diphosphate GDP, in a reaction catalyzed by succinyl CoA synthetase (succinate thiokinase) • Subsequently, the g-phosphoryl group can be readily transferred from GTP to ADP to form ATP, in a reaction catalyzed by nucleoside diphosphokinase (an energy neutral reaction): DGo’ = 0 Step 5: Succinyl-CoA Synthetase Mechanism 1. 2. 3. 4. Exergonic thioester hydrolysis is coupled to endergonic substratelevel phosphorylation of GDP to GTP by formation of several ‘highenergy’ compounds which conserve the initial thioester energy. Phosphorolysis (Pi is nucleophile) of high-energy thioester to form highenergy succinyl-phosphate Transfer phosphate to His to form high energy covalent phospho-His intermediate and succinate Substrate-Level Movement of phosphorylated His near to GDP Phosphorylation Transfer phosphate to GDP to form GTP Phosphorolysis Know Mechanism and Energy Transformations Regeneration of Oxaloacetate Introduce oxygen as -OH, which is then transformed into a C=O 6 7 Oxidation Hydration 8 Oxidation Remaining three reactions of the citric acid cycle (steps 6, 7 & 8), all involve 4C compounds and constitute the final stage of the citric acid cycle - the regeneration of oxaloacetate. 1. Oxaloacetate is regenerated allowing another round of the citric acid cycle to occur 2. More energy is extracted by two more oxidations, in the form of one FADH2 and one NADH Many Metabolic Enzymes Require Cofactors Holoenzyme = Cofactor + Apoenzyme Holoenzyme only has biological activity when cofactor is bound. Cofactor (or coenzyme) = small non-amino acid molecule required for the catalytic activity of an enzyme. Often derived from dietary vitamins and minerals. Cofactor can be an organic molecule, metal ion, or organometallic complex. Cofactors can be either: • Cosubstrate = small organic molecule that associates only transiently with an enzyme. Later associates with another enzyme. • Prosthetic Group = a molecule that is permanently and tightly bound to an enzyme (often, but not always covalently). NADH and FADH2 are MOBILE electron carriers essential for metabolism! • NAD+ and NADP+ shuttle reducing equivalents throughout metabolism, are derived from niacin. • They are cosubstrates for dehydrogenase enzymes. • Once reduced, they transport electrons released by fuel oxidation (glucose metabolism) into the mitochondrial electron transport chain for ATP synthesis. NADH: Activated Carrier of Electrons for Fuel Oxidation NADH is an activated carriers of electrons during fuel oxidation: Reactive site • Oxidation of ROH to RCHO or RCOR’ • Reduction of NAD+ to NADH NADH – is used in catabolic reactions uses free energy of metabolite oxidation to generate ATP, the cell’s “energy currency” NADPH – in anabolic reactions is used as “second energy currency = reducing power” for driving biosynthesis reactions In the oxidation of a substrate, the nicotinamide ring of NAD+ accepts a hydrogen ion and two electrons, which are equivalent to a hydride ion (H:-) NAD+ + 2e- + H+ NADH NADH Oxidation and Reduction: H2 Removal & Addition R H A R O OH R R Oxido-reductase Overall reaction: H H CONH2 + N R • • • • NAD+ H H R C R' OHH Base : CONH2 + N R NADH + R C R' + H O Dehydrogenases TRENDS: (H:-) hydride ion (H+ + 2e-) can be transferred to NAD+ (NADP+) or flavin Driving force in the “forward” direction is electrostatic (charge neutralization), attraction of hydride (negative charge) and pyridinium moiety of cofactor (which has a positive charge) Driving force in “reverse” direction is the resonance gained when the pyridinium becomes aromatic These two driving forces balance one another so that the reaction can be “pushed” one way or the other by the protein structure: NAD+ ⇆ NADH reaction can occur in both directions ! Roles of two redox cofactors NAD+ & FAD in metabolism • Both NAD+/NADH and FAD/FADH2 must undergo reversible redox reactions as they function in electron transfer by accepting electrons, then passing them on to other electron carriers. Thus they are regenerated and reused in additional metabolic cycles. 1. When is FAD used instead of NAD+ as an oxidizing agent in metabolic reactions? • In catabolism, NAD+ functions biochemically in quite exergonic oxidation of alcohols to aldehydes or ketones, oxidation of these to carboxylic acids, and reactions involving decarboxylations (both a and b ). • In catabolism, FAD functions biochemically to oxidize alkanes (such as succinate) to alkenes (such as fumarate), a simple dehydrogenation. 2. When is NADP+ used instead of NAD+ in metabolic reactions? • NADPH/NADP+ are used in anabolic (reductive) pathways (biosynthesis) Step 6: Sucinnate Dehydrogenase: oxidation Succinate + FAD Fumarate + FADH2 • Stereospecific dehydrogenation of 4C succinate to 4C fumarate • Metabolite oxidation, since loss of H2 OUT IN • The FADH2 produced by the oxidation of succinate does not dissociate from the enzyme, but electrons are transferred directly from enzyme-bound FADH2 into the electron transport chain. • Succinate dehydrogenase (complex II) is an integral membrane protein containing an iron-sulfur prosthetic group. The protein is attached to the inner mitochondrial membrane (IMM) and feeds electrons directly into the electron-transport chain, the link between the citric acid cycle and ATP formation (Chapter 18 – Oxidative Phosphorylation). Step 6: Sucinnate Dehydrogenase: Succinate + FAD Fumarate + FADH2 • FAD is reduced as succinate is oxidized. • This is the only membrane-bound enzyme of the citric acid cycle, with a covalently linked prosthetic group (which is unusual). • This is also the only step in oxidative glucose catabolism to use FAD. 1. FAD is a dinucleotide linked to Adenine by two phsophate groups. 2. Note FAD, NAD+, CoA and ATP all contain a adenosine monophosphate unit (AMP, in red) • Humans must obtain riboflavin in their diets as vitamin B2 • The oxidative decarboxylation of pyruvate (PDHC) and the sequence of reactions in the citric acid cycle all take place within the matrix (inner compartment) of mitochondria. Step 7: Fumarase hydration 4C Fumarate 4C Malate • Fumarase catalyzes hydration of fumarate to malate • Reaction involves addition of H2O (OH-/H+) across a double bond, via a carbanion transition state • Note this reaction is stereospecific as a new chiral center is produced in malate forms only L-malate (not D-malate) Step 8: Malate Dehydrogenase oxidation Malate + NAD+ Oxaloacetate + NADH • oxidize 2o alcohol at C2, to a ketone to regenerate oxaloacetate • produce third NADH in one turn of citric acid cycle C2 1. How many turns of the citric acid cycle will result from the complete oxidative catabolism of one glucose molecule? 1 glucose 2 pyruvate 2 Acetyl CoA (+ 2CO2) Regeneration of Oxaloacetate • The last 3 reactions of the citric acid cycle constitute a metabolic motif that is also used in fatty acid synthesis and degradation as well as in the degradation of some amino acids (see end of Chapter 15). • A methylene group (CH2) is converted into a carbonyl group (C = O) in three steps: an oxidation, a hydration, and a second oxidation reaction Oxidation forming FADH2 Hydration Oxidation forming NADH The Citric Acid Cycle Problems: Wkbk SelfTest #8, (Wkbk Pbm # 5) 1. Given the biochemical intermediates of the pyruvate dehydrogenase reaction and the citric acid cycle shown, answer the following questions. (a) Name the intermediates A and B A= B= (b) In isocitrate, which atoms come 1 2 from acetyl CoA? 8 B 2 7 3 6 5 4 A (c) Which reaction is catalyzed by a-ketoglutarate dehydrogenase? (d) Which enzyme catalyzes step 1? (e) Which reactions are oxidations? Name the enzyme that catalyzes each of them. The Citric Acid Cycle Problems: Wkbk SelfTest #8, (Wkbk Pbm # 5) 1. Given the biochemical intermediates of the pyruvate dehydrogenase reaction and the citric acid cycle shown, answer the following questions. (f) At which reaction does substratelevel phosphorylation occur? Name the enzyme and the products of this reaction. 1 8 2 B 2 (g) Which of the reactions require an FAD cofactor? Name the enzyme (h) Indicate the decarboxylation reactions and name the enzymes. 7 3 6 5 4 A http://www.wiley.com/college/fob/quiz/quiz16/16-2.html The Citric Acid Cycle Problems: 1. Which of the following best describes the net organic products formed during the oxidation of ONE molecule of glucose to six molecules of CO2 during glucose catabolism (including glycolysis, pyruvate dehydrogenase complex, and citric acid cycle reactions)? A) B) C) D) E) 4 ATP + 8 NADHmatrix + 2 NADHcytosol + 2 FADH2 6 ATP + 10 NADH 4 ATP + 8 NADH + 2 FADH2 2 FADH2 + 8 NADHmatrix + 2 NADHcytosol 4 ATP + 10 NADHmatrix + 2 FADH2 TCA Provides Intermediates for Biosynthesis As a major metabolic hub of the cell, the citric acid cycle is central pathway in catabolism in oxygenated environment (aerobic), also provides intermediates for biosynthesis Anabolism Examples: Most of the carbon atoms in porphyrins come from succinyl CoA. • Many of the amino acids are derived from a-ketoglutarate & oxaloacetate. • Citrate is a starting material for fatty acid and cholesterol biosynthesis. • The important point now is that citric acid cycle intermediates must be replenished if any are drawn off for biosynthesis. • Suppose that a lot of oxaloacetate is converted into amino acids for protein synthesis and, subsequently, the energy needs of the cell rise. • Citric acid cycle will operate to a reduced extent unless new oxaloacetate is formed, because acetyl CoA cannot enter the cycle unless it condenses with oxaloacetate. Even though oxaloacetate is recycled in TCA, a minimal level must be maintained to allow the citric acid cycle to function. Amphibolic Functions of the Citric Acid Cycle 1. Do anabolic reactions require free energy to occur? 2. For catabolism of Acetyl CoA, are the cellular concentrations of OAA and Acetyl CoA the same? • ________________ amounts of TCA intermediates are needed to maintain the degradative (catabolic) function of the cycle, with release and conservation of free energy. 3. Is there NET production of OAA during the Citric Acid cycle? TCA is both catabolic and anabolic http://www.wiley.com/college/ fob/quiz/quiz16/16-15.html • In a reaction outside TCA, pyruvate (3C) and CO2 (1C) can combine to replenish 4C oxaloacetate. This rxn is catalyzed by pyruvate carboxylase. Regulation of the Citric Acid Cycle High Energy Charge: large [ATP]/[AMP] ratio Control of the Citric Acid Cycle: • Regulated primarily by the concentrations of ATP and NADH (final products of pathway) • Key control points in TCA are the two reactions with the largest driving force (DG’): isocitrate dehydrogenase a-ketoglutarate dehydrogenase - If cell’s energy charge [ATP]/[AMP] is high (because excess ATP is present) then these two enzymes and the entire TCA pathway is downregulated via _______________________. Regulation of the Citric Acid Cycle Low Energy Charge: small [ATP]/[AMP] ratio During exercise use a lot of ATP, so entire TCA pathway is up-regulated. If high rate of TCA then need more: • Oxaloacetate - produced from pyruvate (by pyruvate carboxylase) • Acetyl CoA - produced from either - pyruvate (by PDHC) or - fatty acids (by b-oxidation pathway) Stereochemistry and the Citric Acid Cycle Oxaloacetate Citrate a-Ketoglutarate • Citrate is prochiral, and aconitase (in step 2) can interact asymmetrically with the substrate and distinguish between its two carboxymethyl groups: C1 of citrate is different from C5 • Enzymes are chiral molecules made up of L-amino acids, so they can catalyze asymmetric reactions! • Only one carboxy methyl will fit in enzyme active site once the COO- and OH groups are bound to the enzyme. 1. If C4 of oxaloacetate is radioactively labeled with 14C, at top C1 of aKG where will the label end up in a-ketoglutarate? Note 2 carbons that go in as acetyl CoA (green) are NOT the two that come out in one turn of TCA. C1 C2 C3 C4* C1* C1* C2 C2 C3 2 C3 C4 C4 C5 C5 at top C1 of aKG Citric Acid Cycle : Isotope Labeling Problems Oxaloacetate CH2 -OOC C O + CH3 1 H2O COO- 1 CH2 2= -OOC C OH 3 -OOC CH2 4 C C O b-decarboxylation CoASH COO- O S CoA COOCH2 C OH 5 COOH C OH 2 3 -OOC C H CH2 CH2 COO- COO- [3a] OCitrate substrate level phosphorylation GTP + CoASH CoA CO2 S C O 4 CH2 CH2 COOC O CH2 [3b] COO- a-Ketoglutarate CH2 CoASH 5 CH2 = Succinyl CoA COO- 1 2 CH2 CH2 COO- 2nd CO2 1. Start with label at C4 of Oxaloacetate. Q: Where is label in a-keto glutarate? 3 COO- 4 CH2 GDP + Pi COO- a-Ketoglutarate Oxalosuccinate OC O CH2 COO- Succinate rotate OAA H2O COOCH2 6 H Succinate COOC CH2 COO- 1 2 3 4 5 COO- a-decarboxylation COOC O O H C H C C H -O CH2 Isocitrate Acetyl CoA 1 2 3 4 5 COOC O CO2 7 HO C H CH2 C -OOC COO- H Fumarate COOMalate 8 1 COO2 O C 3 CH2 4 COO- = COO- 4 CH2 3 -OOC C O 2 1 Oxaloacetate Stereochemistry and the Citric Acid Cycle Succinyl CoA Succinate Fumarate Scrambling of 14C at C4 of succinate, occurs during the succinate dehydrogenase reaction (step 6) • Succinate and Fumarate have 2-fold rotational symmetry (plane of symmetry), so they are NOT prochiral, and terminal atoms are scrambled. Substrate can enter enzyme active site with either terminal atom at bottom. Thus a 14C label on C4 of succinate will be scrambled, and appear at both C1 and C4 of fumarate and malate (for half the molecules of each) Label EITHER at C1* or C4*, NOT both C1 C2 C3 C4* = C1* C2 C3 C4* C1* C2 C3 C4* 2. If C1 (carbonyl) of Acetyl CoA is labeled with 14C, where will the label end up in fumarate & malate after 1 turn of TCA? How many positions in a particular molecule of malate will be labeled?. One C labeled: half molecules labeld at C1 and half labeled at C4 Citric Acid Cycle : Isotope Labeling Problem Oxaloacetate CH2 1 C O -OOC + CH3 #C O b-decarboxylation CoASH COO- H2O COO- 1 CH2 2= -OOC C OH 3 -OOC CH2 4 #C O S CoA COOCH2 C OH COOH C OH 2 CH2 CH2 # COO- [3a] Citrate COO- substrate level phosphorylation a-decarboxylation S C O 4 CH2 CH2 CoASH # COO- # COO- a-Ketoglutarate 5 CH2 1 CH2 2 CH2 3 COO- 4 Succinate 6 C # 2 3 COO- 4 CH2 Succinate H2O # COO- 7 HO C H CH2 C -OOC CH2 symmetric molecule so label # 50% at C1, and 50% at C4 #COO- H #COO- 1 # COO- Succinyl CoA #COO- = CH2 GDP + Pi # either at C1 or at C4, not at both # OC O CH2 CH2 OR Oxalosuccinate GTP + CoASH CoA CO2 COOC O COOC O C C H -O CH2 Isocitrate Acetyl CoA 1 2 3 4 5 O COOC O 1 H C H 2 3 CH2 [3b] # COO- 4 5 a-Ketoglutarate 3 -OOC C H # COO- 5 O- CO2 H Fumarate 8 2. Start with label # (C=O) of at C1 AcetylCoA. Where is label in oxaloacetate? One C labeled: half molecules at C1 and half at C4 1 # COO2 O C 3 CH2 4 COO- # = COO- 4 CH2 3 -OOC C O 2 1 # # # COOMalate Second round of TCAOxaloacetate starts with OAA labelled on ½ molecules at C1 and ½ at C4 (a) COO #C (d) - O S-CoA # CH3 C O CH3 Indicates new C-C bond # = + CO2 1 # # 2 1 Pyruvate Dehydrogenase Complex 2 # # OAA labeled either at C1* or C4* NOT both 2. Start with label # at C1 (C=O) of AcetylCoA. Half molecules of OAA labeled at C1 and half at C4 8 # # # Q: Which atoms (and to what percent) is OAA labeled? 7 4 # # # # 14C labeling in the Citric Acid Cycle problems 3 The two CO2 that come off during one round of the cycle are NOT the two that go in as Acetyl CoA in that round 6 # 5 Metabolic Fate of Pyruvate 3. If Pyruvate is isotopically labeled at its C3 (methyl) position with 14C, where will the radioactive label appear after one round of the citric acid cycle? C2 & C3 of OAA labeled, 50% label at each C • • • Will any labeled CO2 be released? No, CO2 comes from OAA Answer by drawing out the product of the pyruvate dehydrogenase step, and the key steps in the citric acid cycle from acetyl CoA to oxaloacetate. Indicate the labeled carbon in glucose with an asterisk (*) and trace this label through every metabolite till oxaloacetate. Citric Acid Cycle: Oxaloacetate (4C) Citrate (6C) a-Ketoglutarate (5C) Succinyl-CoA (4C) Succinate (4C) Fumarate (4C) Malate (4C) Oxaloacetate (4C) * (a) (d) COO- S-CoA C C O O = 1 * CH3 3. What if we start with label at C2 (methyl) of AcetylCoA. * Half molecules of OAA labeled at C2 and half at C3 * + CO2 2 * Pyruvate Dehydrogenase Complex CH3 Isotope Labeling Problem: * * * 3 The two CO2 that come off during one round of the cycle are NOT the two that go in as Acetyl CoA in that round * * * Q: Which atoms (and to what percent) is OAA labeled? 7 4 * * * 14C labeling in the Citric Acid Cycle problem 2 1 8 OAA labeled either at C2* or C3* new C-C bond 6 * * 5