* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 19
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biochemical cascade wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Photosynthesis wikipedia , lookup
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biochemistry wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
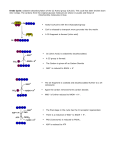
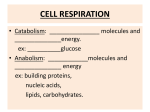
Chapter 19 Bioenergetics; How the Body Converts Food to Energy Metabolism Metabolism: The sum of all chemical reactions involved in maintaining the dynamic state of a cell or organism. • Pathway: A series of biochemical reactions. • Catabolism: The process of breaking down large nutrient molecules into smaller molecules with the concurrent production of energy. • Anabolism: The process of synthesizing larger molecules from smaller ones. Metabolism Metabolism is the sum of catabolism and anabolism. Metabolism Figure 19 .1 Simplified schematic diagram of the common metabolic pathway, an imaginary funnel representing what happens in the cell. Cells and Mitochondria Animal cells have many components, each with specific functions; some components along with one or more of their functions are: • Nucleus: Where replication of DNA takes place. • Lysosomes: Remove damaged cellular components and some unwanted foreign materials. • Golgi bodies: Package and process proteins for secretion and delivery to other cellular components. • Mitochondria: Organelles in which the common catabolic pathway takes place in higher organisms; the purpose of this catabolic pathway is to convert the energy stored in food molecules into energy stored in molecules of ATP. A Rat Liver Cell • Figure 19.2 Diagram of a rat liver cell, a typical higher animal cell. A Mitochondrion • Figure 19.3 Schematic of a mitochondrion cut to reveal the internal organization. The Common Metabolic Pathway • • The two parts to the common catabolic pathway: • The citric acid cycle, also called the tricarboxylic acid (TCA) or Krebs cycle. • Electron transport chain and phosphorylation, together called oxidative phosphorylation. Four principal compounds participating in the common catabolic pathway are: • AMP, ADP, and ATP: agents for the storage and transfer of phosphate groups. • NAD+/NADH: agents for the transfer of electrons in biological oxidation-reduction reactions. • FAD/FADH2: agents for the transfer of electrons in biological oxidation-reduction reactions. • Coenzyme A; abbreviated CoA or CoA-SH: An agent for the transfer of acetyl groups. Adenosine Triphosphate (ATP) ATP is the most important compound involved in the transfer of phosphate groups. • ATP contains two phosphoric anhydride bonds and one phosphoric ester bond. Adenosine Triphosphate (ATP) • Hydrolysis of the terminal phosphate (anhydride) of ATP gives ADP, dihydrogen phosphate ion, and energy. • Hydrolysis of a phosphoric anhydride liberates more energy than the hydrolysis of a phosphoric ester. • We say that ATP and ADP each contain high-energy phosphoric anhydride bonds. • ATP is a universal carrier of phosphate groups. • ATP is also a common currency for the storage and transfer of energy. NAD+/NADH • Nicotinamide adenine dinucleotide (NAD+) is a biological oxidizing agent. + The plus sign on NAD represents the positive charge on this nitrogen O CNH2 O - O-P-O-CH2 O AMP H N+ O H H H HO Nicotinamide; derived from niacin OH a b-N-glycosidic bond NAD+/NADH • NAD+ is a two-electron oxidizing agent, and is reduced to NADH. • NADH is a two-electron reducing agent, and is oxidized to NAD+. The structures shown here are the nicotinamide portions of NAD+ and NADH. • NADH is an electron and hydrogen ion transporting molecule. FAD/FADH2 • Flavin adenine dinucleotide (FAD) is also a biological oxidizing agent. O Riboflavin H3 C N H3 C N N N H Flavin O CH2 H C OH H C OH H C OH CH2 O O=P-O-AMP O- Ribitol FAD/FADH2 • FAD is a two-electron oxidizing agent, and is reduced to FADH2. • FADH2 is a two-electron reducing agent, and is oxidized to FAD. • Only the flavin moiety is shown in the structures below. Coenzyme A • Coenzyme A (CoA) is an acetyl-carrying group. • Like NAD+ and FAD, coenzyme A contains a unit of ADP. • CoA is often written CoA-SH to emphasize the fact that it contains a sulfhydryl group. • The vitamin part of coenzyme A is pantothenic acid. • The acetyl group of acetyl CoA is bound as a high-energy thioester. Coenzyme A • Figure 19.7 The structure of coenzyme A The business end is the -SH (sulfhydryl) group at the left end. Citric Acid Cycle Krebs Cycle Citric Acid Cycle • Figure 19.9 A simplified view of the citric acid cycle showing only the carbon balance. The fuel is the twocarbon acetyl group of acetyl CoA. With each turn of the cycle two carbons are released as CO2. Citric Acid Cycle Step 1: The condensation of acetyl CoA with oxaloacetate: • The high-energy thioester of acetyl CoA is hydrolyzed. • This hydrolysis provides the energy to drive Step 1. • Citrate synthase, an allosteric enzyme, is inhibited by NADH, ATP, and succinyl-CoA. Citric Acid Cycle Step 2: Dehydration and rehydration, catalyzed by aconitase, gives isocitrate. CH2 -COOHO C-COO - CH2 -COO -H2 O CH2 -COO- C-COO - CH-COO H2 O Aconitase CH2 -COOH C-COO HO CH-COO - Citrate Aconitate Isocitrate • Citrate and aconitate are achiral; neither has a stereocenter. • Isocitrate is chiral; it has 2 stereocenters and 4 stereoisomers are possible. • Only one of the 4 possible stereoisomers is formed in the cycle. Citric Acid Cycle Step 3: Oxidation of isocitrate to oxalosuccinate followed by decarboxylation gives a-ketoglutarate. CH2 -COOH C-COO HO CH-COO Isocitrate NAD+ NADH + H+ isocitrate dehydrogenase CH2 -COOH C-COOO C-COOOxalosuccinate CO2 CH2-COO H C-H O C-COOa-Ketoglutarate • Isocitrate dehydrogenase is an allosteric enzyme; it is inhibited by ATP and NADH, and activated by ADP and NAD+. Citric Acid Cycle Step 4: Oxidative decarboxylation of a-ketoglutarate to succinyl-CoA. CoA-SH - CH2 -COO CH2 O C-COO- a-Ketoglutarate NAD + NADH a-ketoglutarate dehydrogenase complex - CH2 -COO CH2 + CO 2 O C SCoA Succinyl-CoA • The two carbons of the acetyl group of acetyl CoA are still present in succinyl CoA. • This multienzyme complex is inhibited by ATP, NADH, and succinyl CoA. It is activated by ADP and NAD+. Citric Acid Cycle • Step 5: Formation of succinate. - CH2 -COO CH2 succinyl-CoA CH -COO synthetase 2 + GDP + Pi + GTP + CoA-SH - O C SCoA CH2 -COO Succinyl-CoA Succinate • The two CH2-COO- groups of succinate are now equivalent. • This is the first and only energy-yielding step of the cycle. A molecule of GTP is produced. Citric Acid Cycle • Step 6: Oxidation of succinate to fumarate. • Step 7: Hydration of fumarate to L-malate. Malate is chiral and can exist as a pair of enantiomers; It is produced in the cycle as a single stereoisomer. Citric Acid Cycle • Step 8: Oxidation of malate. • • Oxaloacetate now can react with acetyl CoA to start another round of the cycle by repeating Step 1. The overall reaction of the cycle is: Citric Acid Cycle Control of the cycle: • Controlled by three feedback mechanisms. • Citrate synthase: inhibited by ATP, NADH, and succinyl CoA; also product inhibition by citrate. • Isocitrate dehydrogenase: activated by ADP and NAD+, inhibited by ATP and NADH. • a-Ketoglutarate dehydrogenase complex: inhibited by ATP, NADH, and succinyl CoA; activated by ADP and NAD+. TCA Cycle in Catabolism The catabolism of proteins, carbohydrates, and fatty acids all feed into the citric acid cycle at one or more points: Oxidative Phosphorylation Carried out by four closely related multisubunit membrane-bound complexes and two electron carriers, coenzyme Q and cytochrome c. • In a series of oxidation-reduction reactions, electrons from FADH2 and NADH are transferred from one complex to the next until they reach O2. • O2 is reduced to H2O. • As a result of electron transport, protons are pumped across the inner membrane to the intermembrane space. Oxidative Phosphorylation • Figure 19.10 Schematic diagram of the electron and H+ transport chain and subsequent phosphorylation. Complex I The sequence starts with Complex I: • This large complex contains some 40 subunits, among them are a flavoprotein, several iron-sulfur (FeS) clusters, and coenzyme Q (CoQ, ubiquinone). • Complex I oxidizes NADH to NAD+. • The oxidizing agent is CoQ, which is reduced to CoQH2. • Some of the energy released in the oxidation of NAD+ is used to move 2H+ from the matrix into the intermembrane space. Complex II • Complex II oxidizes FADH2 to FAD. • The oxidizing agent is CoQ, which is reduced to CoQH2. • The energy released in this reaction is not sufficient to pump protons across the membrane. Complex III • Complex III delivers electrons from CoQH2 to cytochrome c (Cyt c). • This integral membrane complex contains 11 subunits, including cytochrome b, cytochrome c1, and FeS clusters. • Complex III has two channels through which the two H+ from each CoQH2 oxidized are pumped from the matrix into the intermembrane space. Complex IV • Complex IV is also known as cytochrome oxidase. • It contains 13 subunits, one of which is cytochrome a3 • Electrons flow from Cyt c (oxidized) in Complex III to Cyt a3 in Complex IV. • From Cyt a3 electrons are transferred to O2. • During this redox reaction, H+ are pumped from the matrix into the intermembrane space. Summing the reactions of Complexes I - IV, six H+ are pumped out per NADH and four H+ per FADH2. Chemiosmotic Pump To explain how electron and H+ transport produce the chemical energy of ATP, Peter Mitchell proposed the chemiosmotic theory that electron transport is accompanied by an accumulation of protons in the intermembrane space of the mitochondrion, which in turn creates osmotic pressure; the protons driven back to the mitochondrion under this pressure generate ATP. • The energy-releasing oxidations give rise to proton pumping and a pH gradient is created across the inner mitochondrial membrane. • There is a higher concentration of H+ in the intermembrane space than inside the mitochondria. • This proton gradient provides the driving force to propel protons back into the mitochondrion through the enzyme complex called proton translocating ATPase. Chemiosmotic Pump • Protons flow back into the matrix through channels in the F0 unit of ATP synthase. • The flow of protons is accompanied by formation of ATP in the F1 unit of ATP synthase. The functions of oxygen are: • To oxidize NADH to NAD+ and FADH2 to FAD so that these molecules can return to participate in the citric acid cycle. • Provide energy for the conversion of ADP to ATP. Chemiosmotic Pump • The overall reactions of oxidative phosphorylation are: • Oxidation of each NADH gives 3ATP. Oxidation of each FADH2 gives 2 ATP. • Energy Yield A portion of the energy released during electron transport is now built into ATP. • For each two-carbon acetyl unit entering the citric acid cycle, we get three NADH and one FADH2. • For each NADH oxidized to NAD+, we get three ATP. • For each FADH2 oxidized to FAD, we get two ATP. • Thus, the yield of ATP per two-carbon acetyl group oxidized to CO2 is: Other Forms of Energy The chemical energy of ATP is converted by the body to several other forms of energy: Electrical energy • The body maintains a K+ concentration gradient across cell membranes; higher inside and lower outside. • It also maintains a Na+ concentration gradient across cell membranes; lower inside, higher outside. • This pumping requires energy, which is supplied by the hydrolysis of ATP to ADP. • Thus, the chemical energy of ATP is transformed into electrical energy, which operates in neurotransmission. Other Forms of Energy Mechanical energy • ATP drives the alternating association and dissociation of actin and myosin and, consequently, the contraction and relaxation of muscle tissue. Heat energy • Hydrolysis of ATP to ADP yields 7.3 kcal/mol. • Some of this energy is released as heat to maintain body temperature. Other Forms of Energy Figure 19.11 Schematic diagram of muscle contraction. Example How many ATP molecules are generated for each H+ translocated through the ATPase complex? If each mole of ATP yields 7.3 kcal of energy upon hydrolysis, how many kilocalories of energy would you get from 1g of CH3COO-