* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Insulin-Resistance, Browning
Cell encapsulation wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell culture wikipedia , lookup
Signal transduction wikipedia , lookup
Cellular differentiation wikipedia , lookup
Tissue engineering wikipedia , lookup
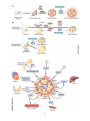
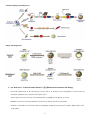
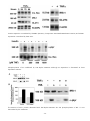
<Seongyong Park> <Research Name> ........................................................................................................................................................................................................ 3 I. Summary .................................................................................................................................................................................................... 21 II. Introduction ................................................................................................................................................................................................. 9 III. 1. Previous Works & Problems of previous works ................................................................................................................. 9 2. Suggested idea & its specific goal, method, expected result & impact ................................................................ 9 Method & Results ..................................................................................................................................................................................... 9 1. Overall methods ................................................................................................................................................................................ 9 2. <1st method & result pair1> ...................................................................................................................................................... 9 3. <2nd method & result pair2> .................................................................................................................................................... 9 IV. Meeting Log ................................................................................................................................................................................................ 9 V. Research Log ............................................................................................................................................................................................... 9 <Patent Name> .......................................................................................................................................................................................................... 10 I. 발명의 명칭 ............................................................................................................................................................................................... 10 II. 기술분야 ...................................................................................................................................................................................................... 10 III. 발명의 배경이 되는 기술 ................................................................................................................................................................... 10 IV. 발명의 내용 ............................................................................................................................................................................................... 10 V. VI. 1. 해결하고자 하는 과제 .................................................................................................................................................................. 10 2. 과제의 해결 수단 ........................................................................................................................................................................... 10 3. 발명의 효과 ....................................................................................................................................................................................... 10 4. 도면의 간단한 설명 ...................................................................................................................................................................... 10 5. 발명을 실시하기 위한 구체적인 내용 ................................................................................................................................. 10 요약서 .......................................................................................................................................................................................................... 10 1. 요약 ....................................................................................................................................................................................................... 10 2. 대표도 .................................................................................................................................................................................................. 10 선행기술자료 조사서 (발명신고)..................................................................................................................................................... 10 1. 산업재산권(관련기술분야 기존 특허 등) 조사 ................................................................................................................. 10 2. 참고문헌, 학회지 등 ..................................................................................................................................................................... 10 VII. 특허 요약 정보 (발명신고) ............................................................................................................................................................. 11 1. 발명의 주요내용 및 특징 ........................................................................................................................................................... 11 2. 적용∙응용분야 ................................................................................................................................................................................... 11 3. 시장성 .................................................................................................................................................................................................. 11 4. 기대효과.............................................................................................................................................................................................. 11 5. 기업화 전망 ....................................................................................................................................................................................... 11 6. 본 기술 적용가능 기업 ............................................................................................................................................................... 11 7. 기타 ....................................................................................................................................................................................................... 11 VIII. Research Log ......................................................................................................................................................................................... 11 <TNF-Alpha Treated Diabetes Mellitus 3T3 L1 (mouse adipose) Cell Model> ............................................................................ 12 I. Summary .................................................................................................................................................................................................... 12 II. Introduction .............................................................................................................................................................................................. 12 1. Previous Works & Problems of previous works .............................................................................................................. 12 2. Suggested idea & its specific goal, method, expected result & impact ............................................................. 17 -1- <Experiment Name> ................................................................................................................................................................................................ 18 I. Summary & Introduction .................................................................................................................................................................... 18 II. Protocol ....................................................................................................................................................................................................... 18 1. <Protocol 1> .................................................................................................................................................................................... 18 2. <Protocol 2> .................................................................................................................................................................................... 18 <Project Name> ......................................................................................................................................................................................................... 19 I. II. III. 최초 연구계획서 ..................................................................................................................................................................................... 19 1. 요약문 .................................................................................................................................................................................................. 19 2. 연구의 필요성 .................................................................................................................................................................................. 19 3. 연구목표 및 내용 ........................................................................................................................................................................... 19 4. 연구의 추진전략 및 방법 ........................................................................................................................................................... 19 5. 연구결과의 활용방안 .................................................................................................................................................................... 19 <2014>년도 연차실적계획서: 기 수행 연구실적 ................................................................................................................... 19 1. 연구 목표 및 내용 ......................................................................................................................................................................... 19 2. 연구수행 결과 .................................................................................................................................................................................. 19 3. 연차 점검의 착안점 및 달성도 ............................................................................................................................................... 19 4. 연구 성과 ........................................................................................................................................................................................... 19 5. 문제점/개선방향/연구변경 사항 .............................................................................................................................................. 19 <2014>년도 연차실적계획서: 차년도 연구계획 ..................................................................................................................... 19 1. 국내외 연구동향 ............................................................................................................................................................................. 19 2. 연구 목표 및 내용 ......................................................................................................................................................................... 19 3. 연구의 추진전략 및 방법 ........................................................................................................................................................... 19 4. 연차 점검의 착안점 ...................................................................................................................................................................... 19 5. 그 밖의 주요 변경사항 ............................................................................................................................................................... 19 IV. Meeting Log ............................................................................................................................................................................................. 19 V. Project Log ................................................................................................................................................................................................ 19 Lab Work ........................................................................................................................................................................................................................ 20 I. <Work Name 1> .................................................................................................................................................................................... 20 II. <Work Name 2> .................................................................................................................................................................................... 20 -2- <Research Idea Development> I. SUMMARY OF BROWNING Gene expression based classification of white adipocyte Browing Agent * 기초 Study - 2000년대 후반 성인에서 근육전구세포 유래 Active Brown Adipose Tissue 발견 - 2010년대 초반 White Adipose Tissue Depot에서 Brown Adipocyte like behavior를 하는 세포 발견 후 Beige Adipocyte로 명명 - Beige Adipocyte는 지방세포 lineage에서 분화하며 WAT와 Beige AT 사이의 Transdifferentiation도 있는 것으로 보고되고 있어 BAT의 분화 촉진 또는 WAT의 Browning이 당뇨 및 비만 치료 기전으로 대두되고 있음. - 현재 Browning Agent들이 다수 보고되고 있으나 해당 Agent들이 어떻게 작용하고 이것이 global gene expression을 어떤 방식으로 Alter하는지에 대해서는 산발적인 Study를 제외하고 Systematic한 Study가 없는 상태임. - 연구실에서 Model 세포로 사용중인 3T3 L1 역시 Beige Adipocyte로 분화가 가능하며 이를 이용한 Beiging 현상에 대한 연구가 근래들어 속속 보고되고 있음 - 이러한 Browning Agent들에는 Dietary Chenmical들도 다수 있는 것으로 보고되고 있어 천연물에서 Browning Agent를 찾을 가능성이 높다고 판단됨. - 따라서 Systematic한 Chemical perturbagen의 global gene expression profiling을 통해 이들 Browning Agent들을 Classify 한다면 이러한 Class에 기반하여 신규 Browning Agent를 Screening 할 수 있을 것으로 판단됨. * Study Outline - Beige adipocyte differentiation/transdifferentiation 관련 문헌 Update - White/beige adipose Tissue gene expression data 수집 - 3T3 L1 기반 White/beige adipocyte gene expression data 수집, 분석 - 3T3 L1 약물처리 expression data 수집, 분석 - 해당 약물처리 data 중에서 browning agent로 알려진 약물 처리 데이터 사이 유사성 분석 - 해당 Agent 처리 후 phenotype 유사성 분석 - 약물 처리 후 expression 유사성 + phenotype 유사성 통합 Scoring - 유사성 Threshold Study - 최종 Threshold를 이용하여 Browning Agent Classfy - 해당 Class들 사이의 약물 유사성 비교 및 신규 Agent 발굴 * Progress - Beige Adipocyte 분화관련 Literature survey - Update 중 - WAT, BAT Expression data 수집 - 3T3 L1 expression data, 약물처리 data 수집 -3- <Research Idea Basic Study> II. WHITE, BROWN, AND BEIGE FAT 1. General Information about Adipocytes - Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. - There are two types of adipose tissue, white adipose tissue (WAT) and brown adipose tissue (BAT), which are also known as white fat and brown fat, respectively, and comprise two types of fat cells. - Although the lineage of adipocytes is still unclear, pre-adipocytes are undifferentiated fibroblasts that can be stimulated to form adipocytes. - Mesenchymal stem cells can differentiated into adipocytes, connective tissue, muscle or bone. - BAT and WAT are histologically distinct types of tissues. - In humans, brown fat is abundant at birth but is rapidly replaced by white adipose tissue (WAT) and is relatively scarce in the adult as an identifiable tissue. Brown fat cells are interspersed within WAT of rodents and humans. Activation of BAT requires 3-adrenergic receptor agonism. -4- 2. WAT Browning - Z:\3-Disease\1-Diabetes-기민난영\TPH(Serotonin)\ 160309 김하일 overview.pptx - Pgc-1, a cold inducible coactivator of PPARγ - PGC-1α is essential for brown fat thermogenesis - But, Pgc-1a is not essential for brown fat differentiation - PRDM16 is selectively expressed in BAT - PRDM16 induces brown adipogenesis - Formation of BAT in WAT depot by PRDM16 expression - KD of PRDM16 in BAT induces skeletal myogenesis - Brown fat and skeletal muscle arise from Myf5-expressing precursors - PRDM16 stimulates adipocyte differentiation in myoblasts - Generation of functional BAT in vivo by expression of PRDM16 and C/EBPβ - Prdm16 is highly expressed in inguinal WAT (iWAT, SC WAT) - Prdm16 stimulates BAT development in iWAT - Mutilocular Ucp1+ cells are prominent in iWAT - Two distinct adipose cell types from iWAT - Beige cells have characteristics of both white and brown cell - Brown fat in adult human are similar to murine beige cells - Sympathetic control of BAT activity in human - Metabolically active BAT in healthy adult human - BAT activity as assessed by PET-CT with 18F-FDG - Different BAT activity in same human under different conditions - Discovery of functional BAT in adult human - Inverse correlation of BAT activity with BMI and body fat - Supercharging Brown Fat to Battle Obesity - Why turning down the thermostat could help win the battle of the bulge? - Factors inducing brown and/or beige adipocytes - Serotonin (5-HT, 5-hydroxytryptamin) - 5-HT receptor - Increased 5-HT production in WAT - Loss of visceral fat by PCPA - PCPA improves insulin sensitivity - PCPA increases energy expenditure - PCPA decreases lipogenesis in eWAT - PCPA induces beige fat formation in iWAT - Increased adaptive thermogenesis in brown fat - Increased size and number of mitochondria in BAT - Increased glucose uptake into brown fat - Tph1 FKO are resistant to HFD - Tph1 FKO increases Ucp1 expression in iWAT - Tph1 AFKO are resistant to HFD - 5-HT plays different roles in adipose tissues - Inhibition of peripheral 5-HT as an anti-obesity treatment strategy -5- -6- <Brown Adipocyte Development> <Beige Fat Biogenesis> 3. Key References - Z:\3-Disease\1-Diabetes-기민난영\TPH(Serotonin)\Fat-Cell-Biology - Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways, Am J Physiol Cell Physiol, 2012 - Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 - PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008). - Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional complex. Nature 460, 1154– 1158 (2009). -7- - Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011). - PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404 (2012). - Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012). - Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 150, 620–632 (2012). - Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Med. 19, 1338-1344 (2013) - Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383 (2013). - EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 504, 163 (2013) - Ablation of PRDM16 and Beige Adipose Causes Metabolic Dysfunction and a Subcutaneous to Visceral Fat Switch. Cell. 156, 304-316 (2014) - Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metabolism, 19(4), 593–604. - Cold-Inducible Zfp516 Activates UCP1 Transcription to Promote Browning of White Fat and Development of Brown Fat. Molecular Cell. - The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature Medicine, 20(12), 1436–1443. - Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nature Medicine. - Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metabolism, 2015 -8- III. DISCOVERY MODEL FOR BROWNING AGENT 1. PPARg agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein - Rosiglitazone induces Browning by inhibiting ubiquitination of PRDM16 in adipocyte, Cell Metabol., 2012 2. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance, Nat. Med. 2015 3. White-to-brown metabolic conversion of human adipocytes by JAK inhibition, Nat. Cell. Biol., 2015 IV. INTRODUCTION Guide: 1000~2000 words + figures 1. Previous Works & Problems of previous works 2. Suggested idea & its specific goal, method, expected result & impact V. METHOD & RESULTS 1. Overall methods 2. <1st method & result pair1> 3. <2nd method & result pair2> VI. MEETING LOG Date & Title: Contents: Date & Title: Contents: VII. RESEARCH LOG Date & Title: Contents: Date & Title: Contents: -9- <Patent Name> I. 발명의 명칭 II. 기술분야 III. 발명의 배경이 되는 기술 IV. 발명의 내용 1. 해결하고자 하는 과제 2. 과제의 해결 수단 3. 발명의 효과 4. 도면의 간단한 설명 5. 발명을 실시하기 위한 구체적인 내용 V. 요약서 1. 요약 2. 대표도 VI. 선행기술자료 조사서 (발명신고) 1. 산업재산권(관련기술분야 기존 특허 등) 조사 기존특허 제목: 특허번호: 특허일자: 본 발명의 어떤 점이 새로운가? (신규성) 본 발명의 진보성 또는 청구범위가 어떻게 다른가? (진보성) 2. 참고문헌, 학회지 등 제목: 저자명: 저널: 연도: 페이지: 본 발명의 어떤 점이 새로운가? -10- 본 발명의 진보성 또는 청구범위가 어떻게 다른가? (진보성) VII. 특허 요약 정보 (발명신고) 1. 발명의 주요내용 및 특징 2. 적용∙응용분야 3. 시장성 4. 기대효과 5. 기업화 전망 6. 본 기술 적용가능 기업 7. 기타 VIII. RESEARCH LOG Date & Title: Contents: Date & Title: Contents: -11- <TNF-Alpha Treated Diabetes Mellitus 3T3 L1 (mouse adipose) Cell Model> I. SUMMARY Guide: ~500 words, Background/Goal/Method/Results/Impact In our lab, we would like to define disease cell model. We used to utilized TNF-alpha treated DM model with 3T3 L1 cell, nobody could justify applicability of the model in our experiments. I would like to justify the utility of the model by comparing experimental results of the cell model from the literatures and recently published another DM model which is treated by FFA (Free Fatty Acids). I have two objectives for this job. First, I’d like to clear controversial results of our glucose uptake experiment. Second, I’d like to use this model for our drug screening platform for glucose uptake enhancing drugs or NPs. II. INTRODUCTION Guide: 1000~2000 words + figures 1. Previous Works & Problems of previous works 1.1 1997, Journal of Biological Chemistry, TNF-alpha-induced IR in 3T3-L1 adipocytes <Abstract> A number of studies have demonstrated that tumor necrosis factor-a (TNF-) is associated with profound insulin resistance in adipocytes and may also play a critical role in the insulin resistance of obesity and noninsulin- dependent diabetes mellitus. Reports on the mechanism of TNF- action have been somewhat contradictory. GLUT4 down-regulation has been implicated as a possible cause of insulin resistance as has been the reduced kinase function of the insulin receptor. Here we examine the effects of tumor necrosis factor on the protein components thought to be involved in insulin-stimulated glucose transport in adipocytes, namely the insulin receptor, its major substrate IRS-1, and the insulin responsive glucose transporter GLUT4. Prolonged exposure (72–96 h) of 3T3-L1 adipocytes to TNF-a causes a substantial reduction (>80%) in IRS-1 and GLUT4 mRNA and protein as well as a lesser reduction (>50%) in the amount of the insulin receptor. Nevertheless, the remaining proteins appear to be biochemically indistinguishable from those in untreated adipocytes. Both the insulin receptor and IRS-1 are tyrosine-phosphorylated to the same extent in response to acute insulin stimulation following cellular TNF- exposure. Furthermore, the ability of the insulin receptor to phosphorylate exogenous substrate in the test tube is also normal following its isolation from TNF--treated cells. These results are confirmed by the reduced but obvious level of insulin dependent glucose transport and GLUT4 translocation observed in TNF-a-treated adipocytes. We conclude that the insulin resistance of glucose transport in 3T3-L1 adipocytes exposed to TNF-a for 72–96 h results from a reduced amount in requisite proteins involved in insulin action. These results are consistent with earlier studies indicating that TNF- reduces the transcriptional activity of the GLUT4 gene in murine adipocytes, and reduced mRNA transcription of a number of relevant genes may be the general mechanism by which TNF- causes insulin resistance in adipocytes. <Main Arguments> 72-96h of TNF-alpha treatment induces 1. Reduction of IRS-1 and GLUT4 mRNA expression (over 80%, in other words, remaining IRS-1 is just 20%) 2. Insulin Receptor reduction (over 50%) 3. Reduced level of insulin dependent glucose transport and GLUT4 translocation 4. Therefore, Insulin resistance phenotype induced by reduced glucose transport receptor and IRS-1 -12- <Scientific Justification> Tumor necrosis factor-a (TNF-a) secretion from activated macrophages has been recognized as one of the initial responses in T2DM disease states. TNF-alters protein and lipid metabolism in adipose tissue and in skeletal muscle. In vivo studies have demonstrated that the adipose tissue of obese insulin-resistant rodents (10) and obese humans (11, 12) has a significant increase in TNF-a production and that neutralization of TNF- in insulinresistant rodents results in an increase in the peripheral uptake of glucose in response to insulin. In hepatocytes, it has been reported that TNF-induces a defect in insulin signaling in a time frame of less than 1 h (16, 17). Low doses (250pM) of TNF- had essentially no effect on basal or insulin-stimulated glucose uptake in 24hrs. There may be a slight decrease in insulin-stimulated glucose uptake in adipocytes exposed to the high doses of TNF- for 24 h (7.8-fold stimulation versus 9.2-fold, untreated). However, at this time, GLUT4 protein is slightly diminished (data not shown), and the basal uptake is slightly elevated (Table I). Thus, we observe no significant compromise in insulin-stimulated glucose transport in adipocytes exposed to relatively short treatments of TNF- and therefore, no lesion in insulin-dependent signal transduction. We also performed a longer time course of TNF-a treatment under conditions previously shown to cause insulinresistant glucose uptake in fully differentiated 3T3-L1 adipocytes (28). As in the cited study, maximal inhibition of insulin-sensitive glucose uptake was not achieved until after 96 h of TNF-a treatment (Table II). Only after 48 h of TNF-exposure do the adipocytes exhibit a significant decrease in insulin-stimulated glucose uptake as compared to untreated cells (Table II). Fig. 1 shows that TNF-a treatment resulted in a slight decrease (~30%) in insulin receptor protein after 96 h. However, there is a very significant decrease in both GLUT4 and IRS-1 levels over this time. Both of these protein decreased by 50–70% after 72 h and were only 20% of controls after 96 h of TNF-a exposure. -13- Protein expression normalized by SCAMPs (Secretory Component-Associated Membrane Proteins) and mRNA expression normalized by beta-actin. Phosphorylation of IR unaffected by TNF-alpha treatment although IR expression is decreased as dose dependent manner The amount of IRS-1 protein decreased with TNF-alpha treatment but the phosphorylation of IRS-1 is not diminished by the treatment. -14- GLUT4 translocation study showed that GLUT4 in plasma membrane decreased with respect to TNF-alpha treatment and translocation of Glut4 in response to insulin does not affected by TNF-alpha treatment. Chronic exposure of 3T3-L1 adipocytes to insulin has long been known to down-regulate insulin receptors (34). More recently, it has been shown that long term exposure of 3T3-L1 fat cells to low doses of insulin causes a significant loss in the cellular GLUT4 content (35) as well as a 70% decrease in IRS-1 protein which, nevertheless, is still tyrosine-phosphorylated in response to insulin (36). In adipocytes, acute TNF-a treatment (15 min) or pretreatment of TNF-a followed by insulin stimulation results in an enhancement of IRS-1 tyrosine phosphorylation and promotes its association with phosphatidylinositol 3kinase (37). Our data indicate that the primary mechanism of TNF-a action is likely to be at the level of regulation of gene expression, the general mode of action for cytokines (32, 33). This hypothesis is supported by previous studies which demonstrate that short (2 h) exposure of 3T3-L1 adipocytes to TNF-a results in a .90% inhibition of GLUT4 transcription in the absence of protein synthesis (28). 10. Hotamisligil, G. S., Shargill, N. S., and Spiegelman, B. M. (1993) Science 259, 87–91 11. Hotamisligil, G. S., Arner, P., Caro, J. F., Atkinson, R. L., and Spiegelman, B. M. (1995) J. Clin. Invest. 95, 2409– 2415 12. Kern, P. A., Saghizadeh, M., Ong, J. M., Bosch, R. J., Deem, R., and Simsolo, R. B. (1995) J. Clin. Invest. 95, 2111–2119 13. Hotamisligil, G. S., Budavari, A., Murray, D., and Spiegelman, B. M. (1994) J. Clin. Invest. 94, 1543–1549 14. Hotamisligil, G. S., Murray, D. L., Choy, L. N., and Spiegelman, B. M. (1994) Proc. Natl. Acad. Sci. U. S. A. 91, 4854–4858 15. Hotamisligil, G. S., Peraldi, P., Budavari, A., Ellis, R., White, M. F., and Spiegelman, B. M. (1996) Science 271, 665–668 16. Jullien, D., Tanti, J.-F., Heydrick, S. J., Gautier, N., Gre´meaux, T., Van Obberghen, E., and Le Marchand-Brustel, Y. (1993) J. Biol. Chem. 268, 15246–15251 17. Feinstein, R., Kanety, H., Papa, M. Z., Lunenfeld, B., and Karasik, A. (1993) J. Biol. Chem. 268, 26055–26058 18. Kanety, H., Feinstein, R., Papa, M. Z., Hemi, R., and Karasik, A. (1995) J. Biol. Chem. 270, 23780–23784 28. Stephens, J. M., and Pekala, P. H. (1992) J. Biol. Chem. 267, 13580–13584 -15- Summary - 96hr 이상의 TNF-alpha 처리가 GLUT4의 발현 억제로 나타나서 adipocyte의 DM phenotype을 형성함. - 48hr 이상에서 이러한 효과가 나타났으며, Insulin pathway는 정상적으로 작동함. 1.2 Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulations is coordinate and independent of protein synthesis. Treating 3T3 L1 with 5 nM TNF-a for 4 hrs yield decrease of GLUT expression. The concentration was 1/20 of 1.1 study. Gene expression in nucleus suppressed by in the present of 0.5 uM of Okadaic acid and 1mM of 8-bromo cAMP Prolonged 5 nM of TNF-alpha treatment induce instability of mRNA expression of GLUT genes. Summary - 72hr 이상의 TNF-alpha 처리가 GLUT4의 발현 억제로 나타나서 adipocyte의 DM phenotype을 형성함. - 이 실험 조건에서는 1.1의 실험 조건보다 TNF-alpha의 농도가 20배 높음 -16- 2. Suggested idea & its specific goal, method, expected result & impact <Comparison between Paper and our Condition> For 3T3 L-1 Paper: 250 pM TNF + 50 nM insulin // Lab: 20 ng/ml TNF + 100 nM insulin Lab에서 쓰고 있는 조건은 20 ng/ml의 농도이며, molar concentration은 780 pM임. 이는 Paper에서 제시한 수치에서 약 3배 정도 높은 concentration임. <Organism, Organ, Cell type specific Glucose Transport Model> 1) Human Adipocyte 2) Rat Adipocyte (3T3 L1) 3) Human Pancreatic Beta Cell 4) Rat Pancreatic Beta Cell 5) Human Muscle Cell 6) Rat Muscle Cell -17- <Experiment Name> I. SUMMARY & INTRODUCTION Guide: 500~1000 words + figures II. PROTOCOL 1. <Protocol 1> 2. <Protocol 2> -18- <Project Name> I. 최초 연구계획서 1. 요약문 2. 연구의 필요성 3. 연구목표 및 내용 4. 연구의 추진전략 및 방법 5. 연구결과의 활용방안 II. <2014>년도 연차실적계획서: 기 수행 연구실적 1. 연구 목표 및 내용 2. 연구수행 결과 3. 연차 점검의 착안점 및 달성도 4. 연구 성과 5. 문제점/개선방향/연구변경 사항 III. <2014>년도 연차실적계획서: 차년도 연구계획 1. 국내외 연구동향 2. 연구 목표 및 내용 3. 연구의 추진전략 및 방법 4. 연차 점검의 착안점 5. 그 밖의 주요 변경사항 IV. MEETING LOG Date & Summary: Contents: Date & Summary: Contents: V. PROJECT LOG Date & Summary: Contents: Date & Summary: Contents: -19- Lab Work I. <WORK NAME 1> Date & Summary: Contents: Date & Summary: Contents: II. <WORK NAME 2> Date & Summary: Contents: Date & Summary: Contents: -20- Idea Stock III. SUMMARY OF DIABETES - CVD Diabetes-CVD Co-Target Identification & Drug Discovery 기술 개발 * 기초 Study - Diabetes 연구동향 및 약물 개발 동향 조사 (Lecture, Review Papers, Clinical Trial 정보) - Diabetes의 major compllication으로 CVD가 가장 큰 문제가 되고 있으나 Clinical Trial 단계에서 기존 CVD 약물의 repurposing 또는 Diabetes Drug과 CVD 약물의 동시 처방 등의 Option 밖에는 현재 개발되어있지 못한 것을 확인함 - 또한 NIH에서 Diabetes-CVD Co-target drug 개발에 Incentive를 주는 것으로 보고되었음. - 따라서 Diabetes-CVD Co-targeted Drug 개발이 필요하나 어떤 Target이 두 질병 사이의 공통기전에 작용하는지 Systematic하게 Study 된 내용이 많이 없음 (이 부분에 대해 추가 조사 필요) * Study Outline - GEO 내 Diabetes와 CVD 관련 환자의 유전자 발현 데이터가 다수 존재함. - 이들 데이터를 가공하여 Diabetes-CVD에서 Co-expression하는 Network를 구축할 수 있음. - 구축 방법은 BIML 2016에서 이인석 교수 연구팀에서 취하는 방법을 활용함 - 구체적으로는 P(Relation)/p(in-TP-Set)이 특정 threshold 이상인 edge를 가지는 network를 Averaging하는 방법임. 이 때 True Positive Set은 Known PPI, Disease Gene 등이 될 것임 - 이렇게 구축한 Network에서 Network Measure를 이용하여 Hub를 찾고, Network Propagation, Context Associated Hub 방법으로 Co-Target을 발굴함. 발굴된 Target의 유의성은 해당 Target과 기존에 알려진 Target 사이의 Distance Measure를 통해 1차적으로 이루어 질 것임. - 발굴된 Target을 Model cell 내에서 (Adipocyte, Pancreatic beta cell, Cardiomyocyte) validation 함 - 해당 Target에 Binding할 약물을 Screening하는 계획은 추후 수립 예정 * Progress - Disease Associate Gene list 확보 & Update 중 - Network Analysis Method - BIML 2016 강의 수강 - 해당 방법론 적용을 위해 해당 내용 Study 중 -21- IV. SUMMARY OF RYGB RYGB에 의한 당뇨 치료기전 발굴 * 기초 Study - 당뇨 관련 Study 중 위 절제술 중 하나인 Rout-en-Y Gastric Bypass Surgery에 의해 80% 이상의 당뇨 환자가 Remission되는 현상이 있다는 사실을 알게됨. - 또한 시술 1년 후에 인슐린 치료를 받던 심한 당뇨환자 62%가 완전히 인슐린 치료를 끊은 것으로 보고된 것을 알게됨. - Clinical Study결과 RYGB 후에 장내 인슐린 조절 호르몬인 Incretin의 농도가 급격히 증가하고, Pancreatic Triglycerol 농도가 감소하였으며, Animal Study에서는 Pancreas beta cell의 proliferation이 증가하고 GLUT4, PPARg 발현, PI3K 인산화 증가, TNF-Alpha mRNA 감소 등의 현상이 관찰되었음. - 식사량 조절, 장내 인슐린 조절 호르몬의 역할이 이러한 extreme phenotype에 대한 기전으로 제시 되었으나 이에 대한 명확한 기전이 밝혀져 있지 못한 상태임. * Study Outline - GEO 내 RYGB 관련 데이터가 많지 않으나 Rat과 Hmuan에서 당뇨 Phenotype을 보이는 환자, Rat에 대해 RYGB를 통해 상태를 개선한 사례가 최소 6건 이상 보고되어 있음 - 이들 데이터를 가공하여 역시 Co-expression Network를 구축하고 GSEA를 통해 Co-express하는 gene이 어떠한 Functional annotation을 가지고 있고, 알려진 Disease Causal gene/SNPs와 어떤 관계가 있는지 확인해보고자 함. - 이들 데이터 셋에서는 RYGB를 받았으나 Diabetes가 remission되지 못한 case가 보고되어 있으므로 이를 분석하여 어떤 차이가 있는지 확인해보고자 함. * Progress - Disease Associate Gene list 확보 & Update 중 - Co-expression Network 분석을 위해서는 Multi platform micro array data analysis, mouse expression, RNA-Seq (optional) 분석이 필요한데 2006년부터 2014년까지 NIH에서 수행한 MAQC Project가 이러한 분석에 필요한 Standard Guideline을 제안하고 있음. 해당 Series Paper를 분석하여 Data 분석 Protocol 을 정리하고자 함. -22- V. SUMMARY OF DRUG SCREENING LOGIC GATES Development of Model Cell that contains logic gates which represent efficacy of drugs. -23-