* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 21 - Immunity
DNA vaccination wikipedia , lookup
Complement system wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adaptive immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
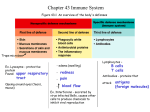
Chapter 21 Nonspecific Body Defenses and Immunity G.R. Pitts, J.R. Schiller, and James F. Thompson, Ph.D. Defense Systems 1. Innate (nonspecific) defenses – External body membranes – Inflammation • Antimicrobial proteins, phagocytes and other cells 2. Adaptive (specific) defenses – T cells and B cells Innate Defense System • Surface Barriers – First line of defense: mechanical and chemical protection 1. Skin 2. Mucosal Membranes • Internal Nonspecific Defenses – 1. 2. 3. 4. 5. Second line of defenses Phagocytes Natural Killer cells (NK lymphocytes) Inflammation Antimicrobial proteins Fever Skin and Mucosal Membranes Mechanical Protection • Epidermis – nose hairs, nails • Mucous membranes - line certain organ systems – mucus prevents drying, traps foreign things – respiratory tract cilia sweep mucus out • Lacrimal apparatus -- tear glands and ducts – wash the eye to dilute microbial growth • Saliva - dilute microbes on the oral cavity • Urine - flow dilutes, and acid pH helps kill, microorganisms • Defecation and vomiting - expel toxins and microbes Skin and Mucosal Membranes Chemical Protection: reduce bacterial growth • Skin – sebum (unsaturated FA’s) forms oily layer – perspiration has fatty acids, salts (NaCl), and mildly acid pH • Lysozyme – in perspiration, tears, saliva, nasal secretions, other tissue fluids – enzyme breaks down bacterial cell walls • Hyaluronic acid – gel-like matrix in most connective tissues – slows the spread of many infectious agents • Gastric juice - stomach nearly sterile due to acid pH, ~2 • Vaginal secretions – mildly acid pH Innate Defense: Phagocytes • Macrophages (derived from monocytes) are the chief tissue phagocytic cells – Free macrophages wander through tissues in search of microbes and cellular debris – Fixed macrophages: Kupffer cells (liver), microglia (brain), dust cells (lungs) • Neutrophils become phagocytic when encountering infectious material • Eosinophils are weakly phagocytic, deploy destructive granules against parasitic worms Mechanism of Phagocytosis • • Chemotaxis Adherence – recognition of external carbohydrates and proteins – Aided by opsonins • • Ingestion Killing and digestion Innate Defense: Natural Killer Cells – Distinct group of large granular lymphocytes (NK lymphocytes = Null Killer lymphocytes) – Nonspecific killers respond to the lack of selfantigens and to the presence of certain surface oligosaccharides – Kill virus-infected body cells and some tumor cells by releasing various defensive molecules – not by phagocytosis – Act before the antigen-specific immune system is activated – Secrete potent chemical signals that enhance the inflammatory response Innate Defense: Inflammation 1. Inflammation • Signs: 1. 2. 3. 4. 5. • Redness Heat Swelling Pain Loss of Function Function: 1. Prevent spread of damage 2. Dispose of pathogens and debris 3. Set stage for tissue repair Inflammation Stage 1: Vasodilation and increased vessel permeability – Macrophages and cells lining the gastrointestinal and respiratory tracts carry Toll-Like Receptors (TLRs) that recognize specific classes of microbes – TLReceptor activation causes cytokine release • promotes inflammation & chemotaxis – Mast cells secrete histamine – Other cells secrete various regulatory factors • Histamine, kinins, prostaglandins, leukotrienes, complement – Cause local vasodilation – Increase capillary permeability resulting in edema http://www.komabiotech.co.kr/technical/review/toll_like_receptor.gif Inflammation: Stage 1 • Edema – increased plasma filtrate seeps into tissue spaces bringing some immune proteins – Helps to dilute harmful substances – Increases supply of oxygen and nutrients needed for metabolism, inflammation and repair – Allows entry of clotting proteins, which reduces the spread of mibrobes Inflammation Stage 2. Phagocyte moblization 1. Leukocytosis-inducing factors: increase neutrophil production 2. Margination (pavementing) 3. Diapedesis (amoeboid movement) 4. Chemotaxis of WBCs • neutrophils – rapid arrival • monocytes – slower arrival Inflammation Stage 3. Tissue repair – Tissue regrowth and repair of damage or scar formation – Pus • dead phagocytes and other WBCs, damaged tissue, and perhaps microbes • if too numerous for effective removal by phagocytes, an abscess may develop Effects of Inflammation • Increased blood flow results in increased local temperature and local cellular metabolism • Increased capillary permeability and phagocytic migration to the injured tissue Innate Defense: Antimicrobial Proteins 1. Attack microorganisms directly 2. Interfere with microbial reproduction • The most important are: 1. Interferons 2. The Complement System 3. Transferrins which bind Fe2+ in plasma, inhibiting bacterial growth Interferons (IFNs) • Produced by most tissue cells when infected by a virus • Diffuses to uninfected cells and binds to surface receptors – stimulates macrophages and natural killer lymphocytes – stimulates production of antiviral proteins which block viral replication – inhibits growth of virally infected cells – suppresses growth of tumor cells • Alpha IFN is used against: – hepatitis C virus – herpes virus (genital warts) The Complement System • 20 plasma and cell membrane proteins that exist as inactive precursors • When activated, the complement system functions to “complement” or enhance certain immune, inflammatory, and allergic responses • Kills bacteria and certain other microbial cell types (our cells normally are protected from complement attack) • Stimulates chemotaxis in leuckocytes • Enhances the effectiveness of both nonspecific and specific defenses Complement Pathways Classical Pathway is triggered by the specific immune system – Requires binding of antibodies to antigens of invading organisms – Complement C1 then binds to the antigen-antibody complexes (complement fixation) Alternative Pathway is triggered by nonspecific interaction among factors B, D, and P, and microbial cell wall polysaccharides (complement fixation) Both pathways involve an enzyme cascade Complement Pathways • Both pathways converge on C3, which cleaves into C3a and C3b • C3b initiates formation of a membrane attack complex (MAC) • MAC causes cell lysis by creating many hundreds of microscopic holes in the cell’s plasmalemma • C3b is also an opsonin Innate Defense: Fever • Pyrogens reset the temperature set-point in the hypothalamus • Inhibits some microbes from growing • Increases body’s metabolic rate, which speeds up immune defenses and tissue repair • Increases effects of antimicrobial substances produced by the immune system • Stimulates liver and spleen to sequester iron and zinc (needed by microorganisms) • High fevers are dangerous Innate Defense System: Review • Surface Barriers 1. Skin 2. Mucosal membranes • Internal Nonspecific Defenses 1. 2. 3. 4. 5. Phagocytes Natural Killer cells (NK lymphocytes) Inflammation Antimicrobial proteins Fever Adaptive Defense • The adaptive immune system: – Acts to immobilize, neutralize, or destroy foreign substances and cells – Amplifies the inflammatory response and activates complement – Is antigen-specific*, systemic, and has memory • *Recognizes specific foreign molecules – Has two interdependent arms • Humoral, or antibody-mediated immunity (AMI) • Cellular, or cell-mediated immunity (CMI) Adaptive Defense • Definitions: – Immunity: the ability of the body to defend itself against specific foreign invaders (molecules or cells) – Immunogenicity: the ability to stimulate proliferation of specific lymphocytes and specific antibody production – Reactivity: the ability of activated lymphocytes and their products, antibodies, etc., to interact with specific antigens Adaptive Defense • Definitions: – Specificity: the antigen triggers focused immune defenses (from particular lymphocytes lineages) that respond only to the antigens of this foreign substance/cell – Memory: the immune system produces clones of specific memory lymphocytes (T & B) which react rapidly when the particular foreign substance/cell is encountered again – Specificity and memory differentiate this system from the nonspecific (innate) defenses Adaptive Defense • Antigen – any substance which provokes specific immune responses • Antigenic determinants – Parts of antigens that trigger the specific immune response – An antigen may be an entire microorganism or only small structures or subregions of large molecules Most “antigens” are complex and express multiple types of antigenic determinants. Chemical Nature of Antigens • “Complete” Ag: complex macromolecules - usually proteins (nucleo-, lipo-, glyco-) -sometimes carbohydrates or lipids – Are immunogenic & reactive • “Incomplete” Ag: smaller molecules (haptens) – react with antibodies but cannot cause an immune response without aid (protein carrier) • e.g., poison ivy, drug allergies Adaptive Defense • Antigen receptor diversity – >1 billion different antigenic determinants are recognized by the body – Genetic recombination shuffles and reorganizes different Ab genes • Major histocompatibility complex antigens (MHC) – unique to each individual’s cells; help in identifying what is self versus foreign – 2 classes of MHC antigens (“markers”) • class I MHC – found on all body cells except RBC's • class II MHC - only on antigen presenting cells (APC’s), thymus cells, and activated T cells Antigen-Presenting Cells (APCs) • APCs phagocytize, process, and present antigens to lymphocytes • APCs do not respond to specific antigens • APCs contribute to coordinating specific immunity – Macrophages – Dendritic (Langerhans) cells – B lymphocytes The major initiators of adaptive immunity are APCs, which actively migrate to the lymph nodes and secondary lymphoid organs and present antigens to T and B cells Class I MHC Proteins • Found on all cells, except RBCs • Recognized by T lymphocytes and APCs • Display peptides from endogenous antigens – Endogenous antigens are: • Associated with body cells • Degraded by proteases and enter the endoplasmic reticulum • Transported through special membrane channels • Bound with MHC class I molecules on the ER membrane • Migrate to the cell membrane as a complex: Ag -- MHC class I molecule MHC Class I Proteins This is a form of Antigen Presentation Cancer cells often do something quite similar to the virus-infected cells. (Foreign MHC Class I Ags are the source of tissue transplant rejections.) MHC Class II Proteins 1. Immune cell identity markers found only on mature B cells, some T cell classes, and antigen-presenting cells 2. MHC Class II proteins are synthesized in the ER 3. A phagosome containing a pathogen (with exogenous antigens) merges with a lysosome 4. MHC Class II proteins migrate into the phagosome where the antigen macromolecules are degraded and particular antigen peptides are bound to the MHC Class II markers 5. Ag-- MHC class II complex then migrates to the cell membrane and displays antigenic peptide for recognition by CD4 TH cells MHC Class II Proteins This is a key function of our APCs in most Ag-specific defenses. Lymphocytes Provide Ag Specificity • B and T lymphocytes develop in bone marrow • Lymphocytes mature and develop immunocompetence (ability to recognize specific antigen) in different locations – B cells mature in the bone marrow and provide Abmediated immunity – T cells mature in the thymus and provide cell-mediated immunity Immunocompetent B or T cells • Naive cells display a unique surface receptor for a specific antigen once mature – Receptor expression occurs before a cell encounters the foreign antigen it may later attack – It is genes, not antigens, that determine which foreign substances our immune system will recognize and resist • Naive cells circulate to secondary lymphoid tissue where they may encounter antigens later – B and T cells become fully functional only after binding with their recognized antigen Immunocompetent T Cells • T cells mature in the thymus under positive and negative selection pressures Survive – Positive selection – outer thymic cortex • Selects functional T cells which become both immunocompetent and potentially self-tolerant • Non-selected cells die via apoptosis Apoptosis Apoptosis – Negative selection – inner thymic cortex • Kill or regulate off T cells that react with self-antigens Immunocompetent B Cells • B cells become immunocompetent and selftolerant in bone marrow – Some self-reactive B cells are killed by apoptosis (clonal deletion) – Some self-reactive B cells can modify their anti-self properties (receptor editing) • Some self-reactive B cells are released from the bone and are inactivated by negative regulation (anergy) Cell-Mediated Immunity • CMI is involved in most aspects of specific immune defense • Three populations of T lymphocytes regulate specific immunity – Helper TH cells which carry CD4+ markers – Suppressor TS cells – Memory T cells • cytotoxic TC cells which carry CD8+ markers destroy tumor cells and virus-infected cells; they also attack transplanted cells and tissues Cell -Mediated Immunity Basic steps 1. Recognition by T lymphocytes of antigen presented by an antigen-presenting cell with matching MHC Class II markers 2. Proliferation and differentiation of T cells once activated 3. Production of clones of identical effector T cells capable of recognizing a specific antigen 4. Appropriate action (help, attack, memory, suppression) from T cell subclones T Cell ActivationStep 1: Antigen Binding and Antigen Presentation T Cell Activation- Step 2: Co-Stimulation • T cells must bind to MHC Class II surface receptors on an APC • After co-stimulation with cytokines, T cells enlarge, proliferate, and form clones • Activated T cells differentiate and perform functions according to their T cell class T Lymphocyte Activity • Primary T cell response usually peaks within a week • T cells then undergo apoptosis within a month • Reduced activity parallels elimination of antigen • This is a negative feedback control • A few Memory T cells remain to respond to any future exposure to the same antigen Helper TH Lymphocytes • Regulatory cells that play a central management role in the immune response • Once primed by APC antigen presentation, TH cells: – Stimulate proliferation of other T cell classes – Stimulate B cells that have already become bound to antigen • There is NO coordinated immune response without TH cell function Helper TH Lymphocytes • TH cells interact directly with B cells that have antigen fragments on their surfaces bound to MHC Class II receptors • TH cells express CD4+ cell identity markers • TH cells stimulate B cells to divide more rapidly and begin antibody formation • B cells may be activated without TH cell help by binding to T cell–independent antigens (certain microbial polysaccharides) • Most antigens, however, require TH co-stimulation to activate B cells • Cytokines released by TH amplify nonspecific defenses Cytotoxic Tc Lymphocytes • TC cells express CD8+ cell identity markers • TC cells, or killer T cells, are the only T cells that can directly attack and kill other cells • They circulate throughout the body in search of body cells that display the antigen to which they have been sensitized • Their targets include: – – – – Virus-infected cells Cells with intracellular bacteria or parasites Cancer cells Foreign cells from blood transfusions (WBCs and platelets) or tissue and organ transplants Cytotoxic Tc Lymphocytes • Bind to self/anti-self complexes on any body cell • Infected or abnormal cells can be destroyed as long as appropriate antigen and co-stimulatory regulators (e.g., IL-2) are present • [In contrast, Natural Killer cells activate their killing machinery when they bind to a different MHC-related cell surface marker on cancer cells, virus-infected cells, and transplanted cells] Cytotoxic Tc Lymphocyte Actions • Secrete perforins which cause cell lysis by creating transmembrane pores • Secrete lymphotoxin which fragments the target cell’s DNA • Secrete gamma interferon which stimulates macrophage attack Suppressor Ts Lymphocytes • TS cells – immune regulatory cells which release cytokines that suppress the activity of both T cells and B cells • Generated when other specific T cell clones are generated • Negative feedback control to bring the body back to normal after the “battle” has been won Antibody-Mediated Immunity • Antigen challenge – the first encounter between an antigen and a naive B lymphocyte • Antigen presentation usually occurs in the spleen or a lymph node, but can occur in any lymphoid tissue • Antigen presentation usually made by a macrophage, but some B cells can react directly against certain bacterial antigens • Binding of the antigen to the B cell’s specific Ag receptor activates the B cell Primary Response Activated B cells grow and divide, forming clones bearing the same antigen-specific receptors and secreting the same antigenspecific Ab • Most clone cells become plasma cells that secrete specific antibodies • Clones that do not become plasma cells become B memory cells that can respond to subsequent exposures to the same antigen Primary Response • Initial B cell differentiation, proliferation, and Ab synthesis requires time after the first Ag exposure • Lag period: 3 to 6 days after antigen challenge • Peak plasma levels of antibody are achieved in ~10 days • Antibody molecules also reach the interstitial fluids, especially where inflammation exists • Antibody levels then decline gradually if there is no additional Ag exposure Secondary Response • Any subsequent exposure to the same antigen • Sensitized memory cells (B and T) respond within hours • Antibody levels peak in 2 to 3 days at higher plasma levels than in the primary response • Activated B subclones generate antibodies that bind with greater affinity • Plasma antibody levels can remain high for weeks to months Primary and Secondary Antibody Responses Immunological Memory • Immunization is possible because memory B cells and memory T cells persist after the initial Ag exposure • with any subsequent exposure, the immune system responds more quickly, forcefully • secondary response - antibodies produced during subsequent exposures are produced in greater quantities and have a greater attraction for antigen Antibodies • Are unique soluble proteins secreted by activated B cells and plasma cells in response to an antigen • Are capable of binding specifically with that antigen • Constitute much of the gamma globulin fraction of plasma proteins • Also called immunoglobulins Basic Antibody Structure • Four polypeptide chains linked together with disulfide bonds • The four chains bound together form an antibody monomer • Each chain has a variable (V) region at one end and a constant (C) region at the other • Variable regions of the heavy and light chains combine to form the antigen-binding site Ag Antibody Structure • Antibodies responding to different antigens have different V regions but the C region is the same for all antibodies in a given antibody class • C regions form the stem of the Y-shaped antibody monomer and determine: – the class of the antibody – the cells and chemicals to which the antibody can bind – how an antibody class functions in eliminating antigens Classes of Antibodies • IgD: monomer attached to the surface of B cells, important in B cell activation • IgM: pentamer released by plasma cells during the primary immune response • IgG: monomer that is the most abundant and diverse antibody in primary and secondary responses; crosses the placenta and confers passive immunity • IgA: dimer that helps prevent attachment of pathogens to mucosal surfaces • IgE: monomer that binds to mast cells and basophils, causing histamine release when activated Antibody Functions • All antibodies form an antigen-antibody (immune) complex • Antibodies do not directly destroy antigen, though they may immobilize or inactivate Ag • Antibodies act as opsonins and tag Ag for immune attack and destruction • Defensive mechanisms triggered by antibodies include neutralization, agglutination, precipitation, opsonization, and complement fixation Antibody Mechanisms of Action 1. Neutralization: Antibodies bind to and block specific sites on viruses or exotoxins, thus preventing these antigens from binding to receptors on tissue cells Antibodies bind to the same determinant on more than one antigen forming antigen-antibody complexes that are cross-linked into large lattices 2. Agglutination: Cellular antigens are cross-linked, causing cell clumping 3. Precipitation: Soluble molecules are cross-linked into large insoluble complexes Antibody Mechanisms of Action 4. Opsonization: Bound Abs facilitate phagocyte adherence 5. Complement Fixation: IgM and IgG antibodies bound to cellular Ags bind complement via the Classical Pathway • • The complement cascade causes chemotaxis, opsonization, phagocytosis and cell lysis Complement activation enhances the inflammatory response Summary of Antibody Actions Figure 21.13 Monoclonal Antibodies • Monoclonal antibodies are purified tissue culture preparations of a specific antibody for a single antigenic determinant which are produced from descendents of a single B cell • Commercially prepared monoclonal antibodies are used: – To provide passive immunity – In research applications – In clinical laboratory testing – In the treatment of certain cancers Adaptive Immunity: Summary • A defensive system with two interdependent arms (CMI & AMI) that uses lymphocytes, APCs, and specific molecules to recognize and destroy foreign substances • Adaptive immune responses depend on the ability of its cells to: – Distinguish foreign from self molecules – React with foreign substances (antigens) by binding to them – Communicate with one another to effect a coordinated protective response specific to those antigens Adaptive Immunity: Summary • To start an immune response, APCs, B and T lymphocytes must recognize foreign antigen • Antigen-Presenting Cells and some B cells recognize and immediately bind to certain antigens in the blood, the extracellular fluid (ECF), or other tissue spaces • More often, B and T cells only recognize antigen (protein fragments) when Ag is presented by the macrophages in combination with MHC Class II surface markers and stimulation is provided by Th lymphocytes Summary of the Immune Response Clinical Classification of Immunity • Active Immunity: the body’s own B and T lymphocytes encounter antigens and produce specific responses against them; immunological memory does occur – Naturally Acquired – response to a microbial or parasitic infection – Artificially Acquired – response to a vaccine of dead or attenuated (weakened) pathogens • Passive Immunity: An outside source of immune cells or molecules is provided to a recipient; immunological memory does not occur; protection ends when the donated materials are naturally eliminated from the body – Naturally Acquired – the mother to her baby via the placenta (IgG) or via lactation (colostrum/milk) (IgM & IgA) – Artificially Acquired – the injection of serum, gamma globulin, or leukocyte transfusion Clinical Classification of Immunity Organ and Tissue Transplants • The four major types of grafts are: – Autograft – graft transplanted from one site on the body to another in the same person – Isograft – graft between identical twins (or clones); individuals with the same genotype – Allograft – graft between individuals that are not identical twins, but belong to same species – Xenograft – grafts taken from another animal species Prevention of Graft Rejection • Donors are selected to minimize differences in MHC Class I antigens = HLA (human leukocyte antigens) – Unnecessary for routine blood transfusions since RBCs lack HLAs • Prevention of rejection is accomplished by using various immunosuppressive drugs – Survival and longevity of grafts have varying success • Immunosuppressive drugs depress the patient’s immune system so it is less effective in defending against pathogens and cancer Pathologies: Immunodeficiencies • Human Immunodeficiency Virus – HIV enters certain cell types by receptor mediated endocytosis • infects primarily helper T cells • attaches to the CD4 protein on cell surface – A retrovirus • carries its genetic material as RNA • inserts its genetic material into host cell DNA with the enzyme reverse transcriptase • cell makes copies of the virus, releases them for further infection – May be carried silently in cells for years, being passed on during ordinary mitosis – Activation of HIV life cycle destroys THelper cells – Weakened immune response to all foreign invaders, benign or aggressive Pathologies: Autoimmune Diseases • Multiple Sclerosis (MS) – myelin sheath (white matter) attacked and destroyed • Myasthenia Gravis – ACh receptors at neuromuscular junction of skeletal muscle attacked and destroyed • Grave’s Disease – thyroid cells’ TSH receptor attacked and stimulated causing excess thyroid hormone (T3 & T4) production • Type I Diabetes - destruction of pancreatic islet cells eliminates insulin secrection Pathologies: Autoimmune Diseases • Systemic Lupus Erythematosus (SLE) – generalized attack on connective tissues and nuclear antigens • Glomerulonephritis - destruction of the glomerular capillaries causes impaired renal function • Rheumatoid Arthritis - destruction of the synovial membranes in joints Pathologies: Cancer • The immune system probably evolved first to respond to cancer cells – when a new cancer cell develops, new surface marker proteins (tumor antigens) often appear – if the immune system recognizes these new surface markers as non-self, it will destroy the cell expressing them – this immune surveillance is most effective in eliminating virus-induced tumor cells because they tend to express viral antigens which are not “self” • Leukemias and Lymphomas – cancers of leukocytes Pathologies: Hypersensitivities • Immediate hypersensitivities (allergies) – First exposure merely sensitizes one to an allergen (penicillin, venoms, dust, mold, pollen, etc.) • APCs digest and inappropriately present the allergen • Subclones of B cells secreting IgE predominate in response • Anti-allergen IgE attaches to mast cells and basophils – Later exposures produce dramatic responses • Antigen binds to IgE on mast cells and basophils • Ag-IgE binding triggers these cells to release much histamine and other inflammatory molecules • Local reactions – swelling, rashes, erythema, itching • Systemic reactions – asthma, anaphylactic shock, death Pathologies: Subacute Hypersensitivities • Caused by IgG and IgM • Occurs 1-3 hr after exposure and lasts 10-15 hr • Cytotoxic reactions – Ab bind to Ag on specific cells causing phagocytosis and complement-activated lysis – May occur after transfusion of mismatched blood • Immune-complex hypersensitivities – Ag’s are widely distributed or insoluble Ag-Ab complexes can’t be removed – Intense inflammation – Severe damage to local tissue – Also involved in autoimmune diseases Pathologies: Delayed Hypersensitivities • Occurs 1-3 days after exposure • Cell-mediated immune response • Causes mild swelling to serious cytotoxic tissue damage (contact dermatitis, e.g., TB skin test, poison ivy, latex gloves, etc.) • [Note: Sometimes allergies may be temporarily transferred by blood or plasma transfusions.] End Chapter 21