* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Toxicant Disposition and Metabolism
Survey
Document related concepts
Human digestive system wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthesis wikipedia , lookup
Western blot wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metabolomics wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
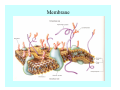
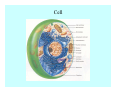
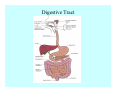
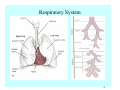
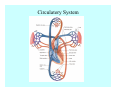
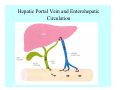
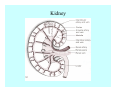
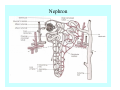
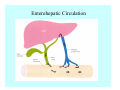
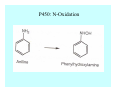
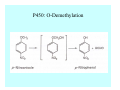
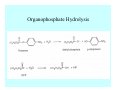
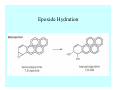
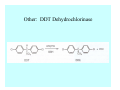
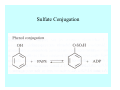
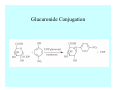
Toxicant Disposition and Metabolism Jan Chambers Center for Environmental Health Sciences College of Veterinary Medicine [email protected] Definitions • Disposition – – – – Absorption—passage across membrane. Distribution—circulation in blood stream. Storage—sequestration. Excretion—elimination from body. • Metabolism – Enzyme-mediated change in chemical structure. – Change in size, configuration, polarity, reactivity. Disposition Absorption • Portals of entry – Digestive tract (oral route of exposure). – Respiratory tract (respiratory route of exposure). – Skin (dermal route of exposure). • Mechanisms of entry – Diffusion across membranes (kinetic energy). – Filtration across membranes (hydrostatic energy). – Active transport (specific carrier protein, metabolic energy). – Endocytosis (pinocytosis, phagocytosis) (receptors, metabolic energy). Membrane Cell Digestive Tract Brush Border Respiratory System • Alveolus Skin Skin Absorption--Summary • Majority of toxicants diffuse through membranes. • Majority of toxicants/xenobiotics of biological interest (e.g., drugs) are lipophilic. • Therefore, a general requirement for toxicant absorption is lipophilicity (non-polar, noncharged). • Toxicant size is generally less relevant than lipophilicity. Distribution • Once absorbed, circulate in the blood stream. • Serum is aqueous medium. • Lipophilic toxicants are not readily dissolved or suspended in the serum. • Bound to serum proteins (e.g., albumin). • Must exit blood and cross membranes to reach biological targets. Distribution serum protein Æ serum Æ capillary membrane Æ interstitial fluid Æ [cell membrane Æ cytosol Æ (organelle membrane Æ interior of organelle)] Æ target molecule (i.e., receptor, enzyme, channel, DNA). Circulatory System Hepatic Portal Vein and Enterohepatic Circulation Storage • Storage sites: – fat stores and fatty tissues (e.g., liver) for lipophilic toxicants; partition into fat; e.g., polychlorinated biphenyls (PCB’s). – bone for divalent cations resembling calcium; active transport using calcium transporter; e.g., lead. • Stored toxicant is biologically inactive until mobilized. Excretion • Major routes: kidney (urine), digestive tract (feces). • Minor routes: respiratory tract, tears, sweat. • Urine and feces are aqueous media. • Lipophilic toxicants cannot partition into urine or feces; therefore, cannot be excreted— bioaccumulation [(e.g., PCB’s , organochlorine insecticides, persistent organic pollutants (POP’s)]. Kidney Nephron Enterohepatic Circulation Metabolism Metabolism/Biotransformation • Chemical alteration to structure. • Enzyme-mediated (enzyme is protein, chemical catalyst, lowers activation energy for reaction). • Outcome: changes physicochemical characteristics of toxicant: – Ability to be stored or excreted (half-life; potential for bioaccumulation). – Reactivity with targets (toxic potential; bioactivation or detoxication). In general: lipophilic (readily absorbed/stored) Æ hydrophilic (readily excreted). Enzyme Reaction Schematic Reaction Pathways Categories of Biotransformation Reactions • Phase 1: adds or uncovers a reactive group. – Makes more polar; more likely to be excreted, but may not be truly water soluble. – More chemically reactive; possibly more toxic. – More likely to undergo Phase 2 metabolism. • Phase 2: adds an endogenous ligand. – Usually makes more polar and usually water soluble. – Usually ligand interacts with reactive group, so decreases toxicity. – May act on parent toxicant or Phase 1 metabolite. Locations of Metabolism • Most active tissue: liver. • Moderately active tissues: kidney, skin, intestine. • Therefore, oral route of exposure leads to greater toxicant metabolism than respiratory or dermal routes. – If toxicant is bioactivated, oral route leads to greater toxicity than other routes. – If toxicant is detoxified, oral route leads to lesser toxicity than other routes Phase 1 Reactions • Oxidation. – Monooxygenases: • Cytochromes P450 (CYP; P450). • Flavin-containing monooxygenases (FMO). – Dehydrogenases: • Alcohol dehydrogenase. • Aldehyde dehydrogenase. • Hydrolysis. – Hydrolases (esterases, amidases). – Hydratases.. • Other. Cytochromes P450 • Enzyme family, with broad substrate specificities. • Most significant of all toxicant oxidation reactions. • Adds one atom of molecular oxygen to substrate, other atom becomes a reactive oxygen species (with potential for oxidative damage within the cell). • Very important in detoxication of many toxicants. • Most important for bioactivations: – carcinogens (e.g., polycyclic aromatic hydrocarbons, PAH’s; benzene; vinyl chloride; aflatoxin). – neurotoxicants (e.g., organophosphate insecticide oxons). – hepatotoxicants (e.g., carbon tetrachloride). P450 Reaction Cycle P450: Epoxidation P450: N-Oxidation P450: Desulfuration/Dearylation P450: O-Demethylation Hydrolysis • Addition of water to break a bond and perhaps the molecule. • Hydrolases (e.g., esterases, amidases): split molecule into two metabolites. • Hydratases: hydrates a bond, but molecule remains intact. • Usually detoxications. Organophosphate Hydrolysis Epoxide Hydration Other: DDT Dehydrochlorinase Phase 2 Reactions • Conjugation reactions, adding an endogenous ligand to a reactive moiety. – Makes more water-soluble and usually detoxifies. • Sulfate. • Glucuronic acid (a sugar). • Glutathione (a peptide). – Makes less water-soluble and more readily absorbed. • Metal methylation; e.g., inorganic to methyl mercury. Sulfate Conjugation Glucuronide Conjugation Glutathione (GSH) Conjugation Reaction Pathways Levels of Enzymes • Vary with age. • Vary with sex. • Inhibition—drug interactions, insecticide synergists. • Induction—alcohol, PAH’s, PCB’s, drugs. SUMMARY • Lipophilic toxicants can get in, but don’t leave. • Phase 1 metabolites more likely to leave but may be highly toxic reactive metabolites. • Phase 2 metabolites readily excreted.































![[4-20-14]](http://s1.studyres.com/store/data/003097962_1-ebde125da461f4ec8842add52a5c4386-150x150.png)















