* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Toxicant Disposition and Metabolism
Human digestive system wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthesis wikipedia , lookup
Western blot wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metabolomics wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
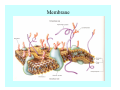
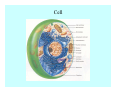
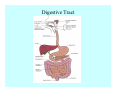
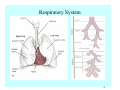
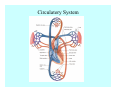
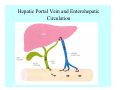
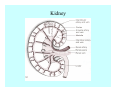
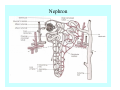
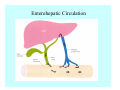
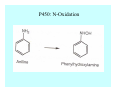
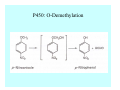
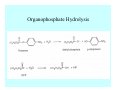
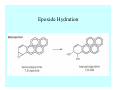
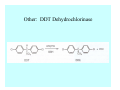
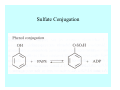
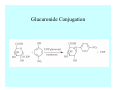
Toxicant Disposition and Metabolism Jan Chambers Center for Environmental Health Sciences College of Veterinary Medicine [email protected] Definitions • Disposition – – – – Absorption—passage across membrane. Distribution—circulation in blood stream. Storage—sequestration. Excretion—elimination from body. • Metabolism – Enzyme-mediated change in chemical structure. – Change in size, configuration, polarity, reactivity. Disposition Absorption • Portals of entry – Digestive tract (oral route of exposure). – Respiratory tract (respiratory route of exposure). – Skin (dermal route of exposure). • Mechanisms of entry – Diffusion across membranes (kinetic energy). – Filtration across membranes (hydrostatic energy). – Active transport (specific carrier protein, metabolic energy). – Endocytosis (pinocytosis, phagocytosis) (receptors, metabolic energy). Membrane Cell Digestive Tract Brush Border Respiratory System • Alveolus Skin Skin Absorption--Summary • Majority of toxicants diffuse through membranes. • Majority of toxicants/xenobiotics of biological interest (e.g., drugs) are lipophilic. • Therefore, a general requirement for toxicant absorption is lipophilicity (non-polar, noncharged). • Toxicant size is generally less relevant than lipophilicity. Distribution • Once absorbed, circulate in the blood stream. • Serum is aqueous medium. • Lipophilic toxicants are not readily dissolved or suspended in the serum. • Bound to serum proteins (e.g., albumin). • Must exit blood and cross membranes to reach biological targets. Distribution serum protein Æ serum Æ capillary membrane Æ interstitial fluid Æ [cell membrane Æ cytosol Æ (organelle membrane Æ interior of organelle)] Æ target molecule (i.e., receptor, enzyme, channel, DNA). Circulatory System Hepatic Portal Vein and Enterohepatic Circulation Storage • Storage sites: – fat stores and fatty tissues (e.g., liver) for lipophilic toxicants; partition into fat; e.g., polychlorinated biphenyls (PCB’s). – bone for divalent cations resembling calcium; active transport using calcium transporter; e.g., lead. • Stored toxicant is biologically inactive until mobilized. Excretion • Major routes: kidney (urine), digestive tract (feces). • Minor routes: respiratory tract, tears, sweat. • Urine and feces are aqueous media. • Lipophilic toxicants cannot partition into urine or feces; therefore, cannot be excreted— bioaccumulation [(e.g., PCB’s , organochlorine insecticides, persistent organic pollutants (POP’s)]. Kidney Nephron Enterohepatic Circulation Metabolism Metabolism/Biotransformation • Chemical alteration to structure. • Enzyme-mediated (enzyme is protein, chemical catalyst, lowers activation energy for reaction). • Outcome: changes physicochemical characteristics of toxicant: – Ability to be stored or excreted (half-life; potential for bioaccumulation). – Reactivity with targets (toxic potential; bioactivation or detoxication). In general: lipophilic (readily absorbed/stored) Æ hydrophilic (readily excreted). Enzyme Reaction Schematic Reaction Pathways Categories of Biotransformation Reactions • Phase 1: adds or uncovers a reactive group. – Makes more polar; more likely to be excreted, but may not be truly water soluble. – More chemically reactive; possibly more toxic. – More likely to undergo Phase 2 metabolism. • Phase 2: adds an endogenous ligand. – Usually makes more polar and usually water soluble. – Usually ligand interacts with reactive group, so decreases toxicity. – May act on parent toxicant or Phase 1 metabolite. Locations of Metabolism • Most active tissue: liver. • Moderately active tissues: kidney, skin, intestine. • Therefore, oral route of exposure leads to greater toxicant metabolism than respiratory or dermal routes. – If toxicant is bioactivated, oral route leads to greater toxicity than other routes. – If toxicant is detoxified, oral route leads to lesser toxicity than other routes Phase 1 Reactions • Oxidation. – Monooxygenases: • Cytochromes P450 (CYP; P450). • Flavin-containing monooxygenases (FMO). – Dehydrogenases: • Alcohol dehydrogenase. • Aldehyde dehydrogenase. • Hydrolysis. – Hydrolases (esterases, amidases). – Hydratases.. • Other. Cytochromes P450 • Enzyme family, with broad substrate specificities. • Most significant of all toxicant oxidation reactions. • Adds one atom of molecular oxygen to substrate, other atom becomes a reactive oxygen species (with potential for oxidative damage within the cell). • Very important in detoxication of many toxicants. • Most important for bioactivations: – carcinogens (e.g., polycyclic aromatic hydrocarbons, PAH’s; benzene; vinyl chloride; aflatoxin). – neurotoxicants (e.g., organophosphate insecticide oxons). – hepatotoxicants (e.g., carbon tetrachloride). P450 Reaction Cycle P450: Epoxidation P450: N-Oxidation P450: Desulfuration/Dearylation P450: O-Demethylation Hydrolysis • Addition of water to break a bond and perhaps the molecule. • Hydrolases (e.g., esterases, amidases): split molecule into two metabolites. • Hydratases: hydrates a bond, but molecule remains intact. • Usually detoxications. Organophosphate Hydrolysis Epoxide Hydration Other: DDT Dehydrochlorinase Phase 2 Reactions • Conjugation reactions, adding an endogenous ligand to a reactive moiety. – Makes more water-soluble and usually detoxifies. • Sulfate. • Glucuronic acid (a sugar). • Glutathione (a peptide). – Makes less water-soluble and more readily absorbed. • Metal methylation; e.g., inorganic to methyl mercury. Sulfate Conjugation Glucuronide Conjugation Glutathione (GSH) Conjugation Reaction Pathways Levels of Enzymes • Vary with age. • Vary with sex. • Inhibition—drug interactions, insecticide synergists. • Induction—alcohol, PAH’s, PCB’s, drugs. SUMMARY • Lipophilic toxicants can get in, but don’t leave. • Phase 1 metabolites more likely to leave but may be highly toxic reactive metabolites. • Phase 2 metabolites readily excreted.































![[4-20-14]](http://s1.studyres.com/store/data/003097962_1-ebde125da461f4ec8842add52a5c4386-150x150.png)








