* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Transport
Survey
Document related concepts
Transcript
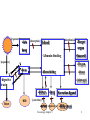
Chapter 3 Transportation and Transformation of Xenobiotics Toxicology-chapter 3 1 •[exposure] •skin •[absorption] •[distribution] blood •lung •Albumin binding •[exposure] •(hazard) •Organ, •liver •dissociating •tissue •digestive tract •(storage) •kidney • feces •Target organ •bile •lung •Secretion ligand •[excretion] •urine •expired gas •Milk,sweat Toxicology-chapter 3 2 Basic Conceptions • Disposition: Absorption, Distribution, metabolism, Excretion (ADME) • Biotransportation: Absorption, Distribution, Excretion • Biotransformation: Metabolism • Elimination: Metabolism, Excretion Toxicology-chapter 3 3 Basic Conceptions • Toxicokinetics: ADME • Toxicodynamics: Interaction, Effects Toxicology-chapter 3 4 Section 1 Absorption Toxicology-chapter 3 5 组织间隙液体 Toxicology-chapter 3 6 Cell Membranes • A phospholipid bilayer with polar head groups on both surfaces. • Proteins or glycoproteins are inserted in or across the bilayer. • The fluid character of membranes is determined largely by the structure and relative abundance of unsaturated fatty acids. Toxicology-chapter 3 7 Transport • Passive transport: simple diffusion; filtration; facilitated diffusion • Active transport: • Cytosis:endocytosis; exocytosis( phagocytosis and pinocytosis ) • Energy needed? • Carrier molecule needed? Toxicology-chapter 3 9 Transport 1. Passive diffusion. Diffusion occurs through the lipid membrane. 2. Filtration. Diffusion occurs through aqueous pores. 3. Special transport. Transport is aided by a carrier molecule, which act as a “ferryboat.” 4. Endocytosis. Transport takes the form of pinocytosis for liquids and phagocytosis for solids. Toxicology-chapter 3 10 Passive diffussion Rate of diffusion D S a Pc (CH CL ) d D is the diffusion coefficient, is primarily dependent on solubility of the toxicant in the membrane and its molecular weight and molecular conformation. Sa is the surface area of the membrane, Pc is the partition coefficient, the relative solubility of the compound in lipid and water, d is the membrane thickness, CH and CL are the concentrations at both sides of the membrane (high and low, respectively). Toxicology-chapter 3 11 Absorption • Absorption: Transfer of a chemical from the site of exposure into the systemic circulation by cross body membranes. • The main sites of absorption are the GI tract, lung and skin, but there are other ways of administration including enteral(肠内的)and parenteral routes. Toxicology-chapter 3 12 Factors Altering the GI Absorption of Toxicants • • • • • pH of the GI 影响酸碱的解离和穿膜扩散 Residency time of compounds Physical properties of chemicals 亲水性和亲脂性 First-pass effect 有些毒物在进入体循环之前首先在胃肠道、肠黏膜细胞和肝脏灭活代谢一部分(主要在肝脏),导致进入体循 环的实际毒物量减少,这种现象称首过消除(first-pass elimination)或第一关卡效应,或首关效应(first-pass effect)。 • Food: ion, milk Toxicology-chapter 3 13 Factors Altering the GI Absorption of Toxicants Enterohepatic circulation • The resident bacterial population can metabolize drugs in the GIT. • If the toxicant survive these microbial and chemical reactions in the stomach and small intestine, it is absorbed in the GIT and carried by the hepatic portal vein to the liver, which is the major site of metabolism. • this activity in the liver can result in detoxification and/or bioactivation. Some drugs and toxicant that are conjugated (e.g., glucuronidation糖脂 化 ) in the liver are excreted via the biliary system back into the GIT. • Once secreted in bile by active transport and excreted from the bile duct into the small intestine, this conjugated toxicant can be subjected to microbial beta-glucuronidase activity that can result in regeneration of the parent toxicant that is more lipophilic than the conjugate. • The toxicant can now be reabsorbed by the GIT, prolonging the presence of the drug or toxicant in the systemic circulation. This is called enterohepatic circulation。 Toxicology-chapter 3 14 Factors altering the lung absorption of toxicants • • • • • Gases and vapors Blood-to-gas partition-coefficient Aerosols and particles Solubility (不同的溶解度在不同部位吸收) Size: 2-5μmainly deposited in the tracheo bronchium Toxicology-chapter 3 15 Factors Altering the Skin Absorption of Toxicants • • • • • Molecular weight Lipid/water solubility Condition of the skin Cutaneous blood flow Solvents Toxicology-chapter 3 17 Section 2 Distribution and Excretion Toxicology-chapter 3 18 Distribution • Distribution: Blood to target organ • Affecting factors: volume of blood flow ; Tissue affinity of xenobiotics • Distribution and Redistribution: Toxicology-chapter 3 19 Distribution • Plasma water • Extracellular water • Intracellular water • Redistribution: organ affinity • Perfusion of tissues • Tissue binding Toxicology-chapter 3 20 Usually after a toxicant or drug is absorbed it can be distributed into various physiologic fluid compartments. Table 6.5 Volume of Distribution into Physiological Fluid Compartments Compartment Volume of Distribution in L/kg Body Weight (Ls/70 kg Body weight) Plasma 0.05(3.5 L) Interstitial fluid 0.18(12.6 L) Extracellular fluid 0.23(16.1 L) Intracellular fluid 0.35(24.5 L) Total body water 0.55(39 L) Storage of Toxicant in Tissues • • • • • Plasma protein Liver and kidney Fat Bone Barrier: blood-brain, placenta Toxicology-chapter 3 22 Excretion • • • • • • • Urinary excretion Fecal excretion Biliary excretion Intestinal excretion Exhalation Milk Sweat and saliva Toxicology-chapter 3 23 TOXICOKINETICS The apparent volume of distribution, Vd • The apparent volume of distribution, Vd , is defined as the volume of fluid required to contain the total amount, A, of drug in the body at the same concentration as that present in plasma, Cp, Toxicology-chapter 3 26 The area under the curve (AUC) • The area under the curve (AUC) is often used to determine how much of the drug actually penetrates the membrane barrier (e.g., skin or gastrointestinal tract) and gets into the blood stream. Absolute Bioavailability, F • The area under the curve (AUC) of the concentration-time profiles for oral or dermal routes is compared with the AUC for IV routes of administration. • absolute bioavailability, F: Clearance • Clearance(清除率) is defined as the rate of toxicant excreted relative to its plasma concentration, Or 单位时间清除的表观分布容积 • Rate of Excretion(消除速度) Toxicology-chapter 3 29 Section 3 Principles of biotransformation of xenobiotics Toxicology-chapter 3 30 Definition • Conversion of lipophilic xenobiotics to water-soluble chemicals by a process catalyzed by enzymes in the liver and other tissues. • In most cases, biotransformation lessens the toxicity of xenobiotics, but many must undergo the process to exert their toxic effects. Toxicology-chapter 3 31 General principles • Broad specificity of xenobiotic biotransforming enzymes such as P450 enzymes. • Biotrasformation versus metabolism • Sterrochemical difference of biotransformation may lead to different metabolites • Phase I and Phase II biotransformation Toxicology-chapter 3 32 Section 4 Phase I and phase II biotransformation Toxicology-chapter 3 33 Phase I biotransformation • Hydrolysis: functional group such as carboxylic 羧基,acid ester, amide, thioester, acid anhydride • Reduction: azo- and nitro-, carbonyl, disulfide, sulfoxide, quinone, dihydropyrimidine • Oxidation: alcohol, aldehyde, ketone, monoamine, aromatization, molybdenum, flavin Key oxidation enzyme: cytochrome P450 Toxicology-chapter 3 34 Cytochrome P450 • Activation of xenobiotics by P450 leads in most cases to detoxication, but some toxicities like tumorigenicity of a chemical depends on its activation. • Some P450 enzymes in human liver microsomes are inducible which usually lowers blood level of the xenobiotics. • Inhibition of P450 falls into 3 categories: the competition between 2 chemicals metabolized by the same P450; by different P450; and by suicide inactivation. Toxicology-chapter 3 35 Cytochrome P450 subtrate(R H) O 2 NADPH H products(R OH) H 2 O NADP Toxicology-chapter 3 36 Phase II biotransformation • Reactions: glucuronidation, sulfonation, acetylation, methylation • Conjugation with glutathione and amino acid can result in a large increase in xenobiotic hydrophilicity to greatly promote the excretion of foreign chemicals • Most phase II biotransforming enzymes are mainly located in the cytosol, and the reactions are much faster than phase I reactions Toxicology-chapter 3 37