* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Essential Question: What is biochemistry
Photosynthetic reaction centre wikipedia , lookup
Isotopic labeling wikipedia , lookup
Drug discovery wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Photosynthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
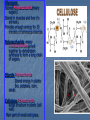
Biochemistry Essential Question: What is biochemistry? Biochemistry- the study of the chemical substances in organisms. Elements: C, H, N, O, P, and S are the most important elements for organisms. Na, K, and Fe are also important. Atoms of elements are almost never found alone, thus they combine to form larger substances called molecules Exs. O2 , F2 or to form compounds Exs. H2O, C6H12O6 . The attraction that hold to atoms together is called a bond. Essential Question: What are Organic Compounds? Organic Compounds: Compounds that contain carbon and hydrogen. Carbon form covalent bonds. Can have up to four bonds. Tend to be very large molecules. Ex. Hemoglobin Made up small units that are covalently bonded together. (freight train) Polymer-large molecule made up by covalently bonding smaller units together. Dehydration Synthesis-process by which a polymer is formed by the removal of water. Hydrolysis-process by which a polymer is broken into smaller units by the addition of water. Four Major Groups of Organic Compounds: Carbohydrates Lipids Proteins Nucleic Acids Carbohydrates(CHO): Contain C, H, O H and O are in a 2:1 ratio Ex. C6H12O6 Usually end in ose. Exs. Sucrose, Maltose. Called simple sugars (monosaccharide) Ex. Glucose Used for energy storage and providing building materials Exs. Glycogen, Lactose, and Cellulose Glycogen: Stored Polysaccharide(many sugars). Stored in muscles and liver (in animals). Provides enough energy for 30 minutes of strenuous exercise. Polysaccharide -many monosaccharides joined together by dehydration synthesis to form a long chain of sugars. Starch- Polysaccharide Stored energy in plants Exs. potatoes, corn, seeds Cellulose-Polysaccharide Builds structure in plants (cell wall) Main part of wood and grass. Types of Sugars • • • • Lactose -milk sugar Fructose- fruit sugar Sucrose-table sugar Maltose- malt sugar Lipids (fats, oils) Long term storage, basic structure of cell membrane, insulation and protection Composed of glycerol(backbone) and 3 fatty acids. Two Types of Lipids: Fatty Acids: Saturated Fatty Acid-every carbon in the chain is bonded to a Hydrogen. Has only single bonds. Exs. meat, butter Unsaturated Fatty Acid- every carbon is not bonded to a Hydrogen. May contain double bonds. Ex. vegetable oil B. Steroids-consist of a ring of carbon atoms Ex. Cholesterol (found only in animal). Cholesterol-----testosterone -----estrogen **Too much cholesterol can lead to atherosclerosis (hardening of arteries). Can lead to heart disease. Proteins: a. More diverse than other organic compounds. (100’s of 1,000’s of different • • • • • • • • • • proteins) b. More complex and larger than other organic compounds. Makes up a large % of the body. (1/2 remaining weight after removal; of water) Functions: Building materials Ex. Collagen and Elastin Transport other substances Ex. Hemoglobin Send chemical messages. Ex. Insulin and thyroxin (hormones) Provide defense (antibodies) Control chemical and metabolic activities Ex. Enzymes control digestion Made up of polymers of Amino Acids (20 A.A.) Exs. Alanine, Tryptophan, Glycine Functions of Proteins Enzymes A. Enzymes: a. Type of protein b. Are organic catalyst Catalyst-chemical that controls the rate of a chemical reaction. Ex: Speeds it up (100 years vs. 10x/sec) c. Reaction specific. Exs. Lipase-lipids maltase-maltose protease-proteins Enzymes D. Every reaction occurring in an organism requires an enzyme. E. Not affected by a chemical reaction F. May be used over and over. G. Enzymes work by the Lock and Key Concept. Lock and Key: Substrate- Material an enzyme works on. Ex. Lactase—lactose. Enzyme-Substrate Complex-Temporary association with the substrate. Active Site-Part of an enzyme that comes in contact with a substrate. Activation Energy-Energy needed to start a reaction. Ex. Match. • Only small amounts of enzymes are needed. Coenzyme-Substances that help enzymes work. Exs. Vitamins and minerals (Zn, Fe, Cu) Scurvy -Deficiency of vitamin C Black and blue spots due to leakage of blood from capillaries. Teeth fall out and vision problems. Enzymes absent that cements the walls of the capillaries . Factors Influencing Enzymes Action: Temperature. a. Optimum temperature is 37 C in humans and 25 C in plants Changes shape of the active site, thus reaction can’t occur. Change in active site is not reversible. Ex. Cooking and egg. pH: a. Optimum pH is 7 Changes in pH, change the shape of enzyme. Nucleic Acids . Nucleic Acids: a. Organic compound b. Made up of nucleotides (1,000) Two Types: DNA(deoxyribonucleic acid) RNA(ribonucleic acid) pH: pH-Measure of how acidic or basic a substance is. pH scale: a. Scale from -14. Below 7-acid. 7 is neutral. 7.1-14-base. Acid-a. Substance that forms H ions in solution. Turns litmus paper red. Tastes sour. Exs. Citric acid, acetic acid Base-a. Substance that forms OH ions in solution. Turns litmus paper blue. Is caustic. Exs. Soap, cleaning supplies.