* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 4 Classes of Large Biological Molecules Carbohydrates Lipids
Survey
Document related concepts
Citric acid cycle wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Gene expression wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Point mutation wikipedia , lookup
Phosphorylation wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Metalloprotein wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Proteolysis wikipedia , lookup
Transcript
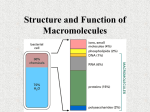
4 Classes of Large Biological Molecules Carbohydrates Lipids Proteins Nucleic Acids Monomer vs. Polymer Most biological molecules are made from smaller subunits called monomers Polymers are formed by covalently bonded monomers Building and Breaking of Polymers Condensation Reaction Hydrolysis Diversity of Macromolecules Polymer arrangement can be compared to the alphabet Most living things use 40-50 most common monomers Arrangement is the most important aspect Carbohydrates Include sugar and the polymers of sugars Monosaccharides Disaccharides Polysaccharides Monosaccharides Molecular Formula: CH2O Glucose: most common monosaccharide Ketose vs. Aldoses Number of carbons dictates naming of simple sugars Most sugars form rings in the presence of water Disaccharides Two monosaccharides joined together by glycosidic linkage Glucose and Fructose = Sucrose (table sugar) Lactose = Glucose and Galactose Polysaccharides Polymers of a few hundred to a few thousand monosaccharides Can be used in storage of E and structural Storage Starch: consists entirely of glucose Joined together by 1-4 glycosidic linkages Allows plants to stockpile E Most animals have enzymes that hydrolyze starch Glycogen Branched version of starch that is used by animals as glucose storage Located in the liver and muscle cells In human, glycogen supply can last about 1 day Structural Polysaccharides: Cellulose Most abundant organic compound on Earth Polymer of glucose, but differs from cellulose Starch vs. Cellulose Starch : 1-4 linkage of alpha glucose Cellulose: 1-4 linkage of beta glucose Interesting fact: humans do not have the enzyme to hydrolyze beta glucose linkages Chitin: the “unused” structural polysac Composes the exoskeleton of arthropods Chitin is soft but hardened with calcium carbonate Fungi also contains chitin Lipids One class of biological molecules that does not contain polymers Common trait: hydrophobic Composed mostly of hydrocarbons Fats Composed of glycerol and fatty acids Glycerol: alcohol with 3 C’s, each possessing a –OH group Fatty acid: hydrocarbon of 16-18 C’s in length, one end has carboxyl group Ester linkage: bond between hydroxyl and carboxyl group Saturated vs. Unsaturated Fat Fat vs. Polysaccharides 1g of fat contain 2X the amount of E as 1g of starch Fat from plants comes from seeds Phospholipids Has two fatty acids attached to a glycerol molecule The 3rd –OH group is attached to a phosphate group (- charge) Show ambivalent properties toward water Steroids Have C skeletons consisting of 4 rings, only variation come in functional groups Cholesterol: precursor from which many other steroids are made Proteins Compose 50% of the dry mass of most cells Some are enzymes; others play roles in structural support, storage, transport, cellular communications, movement, and defense Enzyme Acts as cellular catalyst: selectively speed up reaction w/o being consumed Substance enzyme attaches to is called a substrate Most important Aspect of Proteins A proteins structure defines its function. Change its structure and the protein’s function will change completely. Amino Acid Monomers All proteins are made from 20 different Amino Acids Polymers of amino acids are called polypeptides Protein consists of one or more polypeptides folded into specific configurations General Amino Acid Structure Amino Acid Polymerization Carboxyl group and amino group covalently bond to form a peptide bond N terminus and C terminus Proteins One or more polypeptides precisely folded, twisted, and coiled into a unique shape Amino acid sequence determined 3-D conformation Proteins structure determines its function 4 Levels of Protein Structure 1. Primary 2. Secondary 3. Tertiary 4. Quaternary Denaturation pH, salt concentration, and temperature can alter a protein’s shape Sometimes proteins can renature Central Dogma of Biology DNA à RNA à Protein Nucleic Acids Determines the primary structure of a polypeptide Gene: codes for an amino acid sequence in a polypeptide DNA Arranged in chromosomes that contain one long DNA molecule consisting of several hundred or thousand genes Bound to the nucleus RNA: smaller pieces of nucleic acids that directly make the proteins for the DNA codes 1. Transfer 2. Messenger 3. Ribosomal Nucleic Acid Structure Ring Structure of Nitrogenous Bases Pyrimidine: 1 ring structure; includes cytosine, uracil, thymine Purine: 2 ring structure; includes guanine and adenine