* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Who needs an artificial cornea?
Magnesium transporter wikipedia , lookup
Cell encapsulation wikipedia , lookup
Signal transduction wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
List of types of proteins wikipedia , lookup
Tissue engineering wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
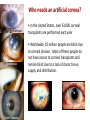
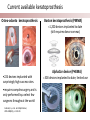
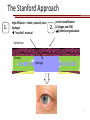
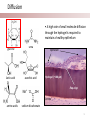
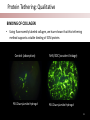
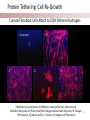
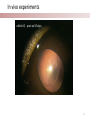
Stanford Cornea Project 1 Laura Hartman, Dale Waters, Rachel ParkeHouben, Curtis W. Frank Stayce Beck, Luo Luo Zheng, Yuhua Hu Jennifer Cochran Resmi Charalel, Phil Huie, Vijay Vanchinathan Roopa Dalal, Michael Carrasco, Jaan Noolandi Christopher N. Ta 2 Who needs an artificial cornea? trachoma trachoma corneal ulcer corneal ulcer • In the United States, over 33,000 corneal transplants are performed each year • Worldwide, 10 million people are blind due to corneal disease. Most of these people do not have access to corneal transplants and remain blind due to a lack of donor tissue supply and distribution. Current available keratoprosthesis Osteo-odonto keratoprosthesis Boston keratoprosthesis (PMMA) ≈1,200 devices implanted to date (still requires donor corneas) AlphaCor device (PHEMA) •224 devices implanted with surprisingly high success rates. ≈ 300 devices implanted to date; limited use •requires complex surgery and is only performed by a select few surgeons throughout the world Falcinelli, G., et al. Arch Ophthalmol, 2005. 123(10): p. 1319-29. 4 Properties of an Artificial Cornea • • • • • • Biocompatible Optically clear centrally Nutrient permeable Mechanically strong Surface epithelialization Peripheral tissue integration CAD model acknowledgement: L. Kourtis, Stanford Dept. of Mechanical Engineering The Stanford Approach 1. high diffusion + stable, optically clear hydrogel “invisible” material 2. protein modification (Collagen and EGF) Epithelium grows back Epithelium Stroma hydrogel hydrogel 6 Material Single Network Double Network • no chemical linkage • two interpenetrating networks (IPNs) • highly improved mechanical properties Single Network Double Network polymerization of 2nd network 1st network swollen in monomeric building blocks of 2nd network 7 J.P. Gong, et al., Advanced Materials 2003 Mechanical Stability 1st network: Poly(ethylene glycol) (PEG) 2nd network: Poly(acrylic acid) (PAA) water content: ~90% IPN 88 77 tunable material IPN Maximum Tensile Stress (MPa) 66 • mechanical stability (contact lens vs. inlay) 00 PEG 3.4K PAA DN (PEG 3.4K-PAA) 1 PEG 8.0K PAA DN (PEG 8.0K-PAA) PEG 14K PAA IPN PEG (14kDa) 11 PAA 22 PEG (8kDa) 33 PAA 44 PEG (4.6kDa) 55 PAA Maximum Tensile Stress [MPa] 9 • pore size: diffusion (nutrient vs. drug delivery) • longterm stability (implant vs. tissue scaffold) DN (PEG 14K-PAA) 8 Diffusion • A high rate of small molecule diffusion through the hydrogel is required to maintain a healthy epithelium glucose urea lactic acid ascorbic acid Epithelium Hydrogel (~100 μm) Flap edge Stroma amino acids sodium bicarbonate 9 Protein Tethering: Cell Re-Growth • no de-swelling of the gel • washing in buffer possible • no denaturation of proteins 10 Protein Tethering: Qualitative BINDING OF COLLAGEN • Using fluorescently-labeled collagen, we have shown that this tethering method supports a stable binding of ECM protein. Control (adsorption) PEG Diacrylamide Hydrogel NHS/EDC (covalent linkage) PEG Diacrylamide Hydrogel 11 Protein Tethering: Cell Re-Growth Corneal Fibroblast Cells Attach to ECM-Tethered Hydrogels A) control B D C D E Phalloidin (red) and Nuclear (DAPI(blue)) staining of primary rabbit corneal fibroblast cells grown on PEGacrylate/PAA Hydrogel tethered with A)Control, B) Collagen, C)Fibronectin, D)Laminin and E) 1:1 mixture of collagen and Fibronectin. 12 In vivo experiments rabbit # 2 - post-op 50 days 13 Future Work MATERIAL • Determine diffusion coefficients for other proteins through human cornea • Apply principles to development of artificial cornea • Modify refractive index for inlay application (presbyopia) DEVICE Protein tethering • Optimize the ECM content tethered to the hydrogel • Use time-lapse microscopy to study cell migration on the hydrogel • Addition of enhanced growth factor (EGF) to the protein layer • • • Tissue Integration Fine-tuning is still needed to reduce the pore diameter to 50 – 100 μm Confocal fluorescence microscopy will be used to demonstrate that the channels are interconnected Tether proteins to the channel walls and test for fibroblast growth IN VIVO EXPERIMENTS • Implant hydrogel-onlays/inlays • Implant artificial cornea 14 Funding • National Institutes of Health / National Eye Institute – R01 EY016987 – NIH Grant 5T90 DK070103-03. • • • • • • Singapore Eye Research Institute (SERI) BioX Stanford Office of Technology Licensing Stanford MedScholar Program Fight for Sight Visx 15 16