* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download AP Biology Ch. 9 Cellular Respiration
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Photosynthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Phosphorylation wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
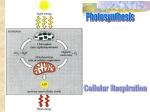
Organisms must take in energy from outside sources. Energy is incorporated into organic molecules such as glucose in the process of photosynthesis. Glucose is then broken down in cellular respiration. The energy is stored in ATP. Fig. 9-2 The Flow of Energy from Sunlight to ATP Light energy ECOSYSTEM Photosynthesis in chloroplasts CO2 + H2O Cellular respiration in mitochondria Organic + molecules O2 ATP ATP powers most cellular work Heat energy Energy in food is stored as carbohydrates (such as glucose), proteins & fats. Before that energy can be used by cells, it must be released and transferred to ATP. Aerobic Cellular Respiration: the process that releases energy by breaking down food (glucose) molecules in the presence of oxygen. Formula: C6H12O6 + 6O2 → 6CO2 + 6H2O +~ 36 ATP Fermentation: the partial breakdown of glucose without oxygen. It only releases a small amount of ATP. Glycolysis: the first step of breaking down glucose—it splits glucose (6C) into 2 pyruvic acid molecules (3C each) Adenosine triphosphate (ATP) “stores” energy in the bonds between its’ 3 phosphates. ATP has the sugar ribose, the nitrogen base adenine, and 3 phosphoric acids. The transfer of electrons during chemical reactions releases energy stored in organic compounds such as glucose. Oxidation-reduction reactions are those that involve the transfer of an electron from one substance to another. • Chemical reactions that transfer electrons between reactants are called oxidation-reduction reactions, or redox reactions In oxidation, one substance loses electrons, or is oxidized In reduction, a substance gains electrons, or is reduced (the amount of positive charge is reduced) Na will easily lose its outer electron to Cl . Why? In this reaction, which atom is oxidized? Which is reduced? Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Redox Reactions of Cellular Respiration In cellular respiration, glucose is broken down and loses its electrons in the process. The glucose becomes oxidized and the Oxygen is reduced. becomes oxidized becomes reduced In cellular respiration, glucose is broken down in a series of steps. As it is broken down, electrons from glucose are transferred to NAD+, a coenzyme When it receives the electrons, it is converted to NADH. NADH represents stored energy that can be used to make ATP NADH passes the electrons to the electron transport chain, a series of proteins embedded in the inner membrane of the mitochondria. The electrons (and the energy they carry) are transferred from one protein to the next in a series of steps. Energy is released a little at a time, rather than one big explosive reaction: H2 + 1/2 O2 2H (from food via NADH) Controlled release of + – 2H + 2e energy for synthesis of ATP 1/ 2 O2 Explosive release of heat and light energy 1/ (a) Uncontrolled reaction (b) Cellular respiration 2 O2 Cellular respiration has three stages: Glycolysis (breaks down glucose into two molecules of pyruvate) The Citric Acid cycle/Kreb’s Cycle (completes the breakdown of glucose) Electron Transport Chain and Oxidative phosphorylation (accounts for most of the ATP synthesis) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings An Overview of Cellular Respiration–Part 1 Electrons carried via NADH Glycolysis Pyruvate Glucose Cytosol ATP Substrate-level phosphorylation An Overview of Cellular Respiration—Part 2 Electrons carried via NADH and FADH2 Electrons carried via NADH Citric acid cycle Glycolysis Pyruvate Glucose Mitochondrion Cytosol ATP ATP Substrate-level phosphorylation Substrate-level phosphorylation An Overview of Cellular Respiration—Part 3 Electrons carried via NADH and FADH2 Electrons carried via NADH Citric acid cycle Glycolysis Pyruvate Glucose Oxidative phosphorylation: electron transport and chemiosmosis Mitochondrion Cytosol ATP ATP ATP Substrate-level phosphorylation Substrate-level phosphorylation Oxidative phosphorylation Substrate-level phosphorylation: Phosphate is added to ADP to make ATP by using an enzyme: Oxidative phosphorylation: Phosphate is added to ADP to make ATP by ATP Synthase—a protein embedded in the mitochondria membrane (requires O2) WAY MORE EFFICIENT!! PRODUCES LOTS MORE ATP! “Glyco”=sugar; “lysis”=to split In this first series of reactions, glucose (C6) is split into two molecules of pyruvic acid (C3). This occurs in the cytoplasm of cells and does not require oxygen. This releases only 2 ATP molecules, not enough for most living organisms. http://highered.mcgrawhill.com/sites/0072507470/student_view0/cha pter25/animation__how_glycolysis_works.htm l Energy investment phase Glycolysis Glucose 2 ADP + 2 P 2 ATP used 4 ATP formed Energy payoff phase 4 ADP + 4 P 2 NAD+ + 4 e– + 4 H+ 2 NADH + 2 H+ 2 Pyruvate + 2 H2O Net Glucose 4 ATP formed – 2 ATP used 2 NAD+ + 4 e– + 4 H+ 2 Pyruvate + 2 H2O 2 ATP 2 NADH + 2 H+ The Citric Acid Cycle (also called the Kreb’s Cycle) completes the breakdown of pyruvate and the release of energy from glucose. It occurs in the matrix of the mitochondria. In the presence of oxygen, pyruvate enters the mitochondria. Before the pyruvate can enter the Citric Acid Cycle, however, it must be converted to Acetyl Co-A. Some energy is released and NADH is formed. Converting Pyruvate to Acetyl CoA: MITOCHONDRION CYTOSOL NAD+ NADH + H+ 2 1 Pyruvate Transport protein 3 CO2 Coenzyme A Acetyl CoA The Acetyl Co-A enters the Citric Acid Cycle in the matrix of the mitochondria. The Citric Acid cycle breaks down the Acetyl Co-A in a series of steps, releasing CO2 It produces 1 ATP, 3 NADH, and 1 FADH2 per turn. The Citric Acid Cycle • • • The Citric Acid cycle (also called the Krebs Cycle) has eight steps, each catalyzed by a specific enzyme The acetyl group of acetyl CoA joins the cycle by combining with oxaloacetate, forming citrate (Citric Acid). The next seven steps decompose the citrate (Citric Acid) back to oxaloacetate, making the process a cycle Oxaloacetate + Acetyl CoA Citric Acid The Citric Acid Cycle: Acetyl CoA CoA—SH NADH +H+ H2O 1 NAD+ 8 Oxaloacetate 2 Malate Citrate Isocitrate NAD+ Citric acid cycle 7 H2O NADH + H+ 3 CO2 Fumarate CoA—SH 6 -Ketoglutarate 4 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 http://highered.mcgrawhill.com/sites/0072507470/student_view0/cha pter25/animation__how_the_krebs_cycle_wor ks__quiz_1_.html • • Each Citric Acid Cycle only produces 1 ATP molecule. The rest of the energy from pyruvate is in the NADH and FADH2. The NADH and FADH2 produced by the Citric Acid cycle relay electrons extracted from food to the electron transport chain. The electron transport chain is in the cristae of the mitochondrion Most of the chain’s components are proteins, which exist in multiprotein complexes The carriers alternate reduced and oxidized states as they accept and donate electrons Electrons drop in free energy as they go down the chain and are finally passed to O2, forming H2O Oxygen is the final electron acceptor. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Electron Transport Chain NADH 50 2 e– NAD+ FADH2 2 e– 40 FMN FAD Multiprotein complexes FAD Fe•S Fe•S Q Cyt b 30 Fe•S Cyt c1 I V Cyt c Cyt a Cyt a3 20 10 2 e– (from NADH or FADH2) 0 2 H+ + 1/2 O2 H2O Electrons are transferred from NADH or FADH2 to the electron transport chain Electrons are passed through a number of proteins to O2 The chain’s function is to break the large freeenergy drop from food to O2 into smaller steps that release energy in manageable amounts http://www.youtube.com/watch?v=xbJ0nbzt 5Kw Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space H+ then moves back across the membrane, passing through channels in ATP synthase Animation: http://sp.uconn.edu/~terry/images/anim/ETS. html ATP synthase uses the exergonic flow of H+ to drive phosphorylation of ATP This is an example of chemiosmosis, the use of energy in a H+ gradient to drive cellular work INTERMEMBRANE SPACE ATP Synthase H+ Stator Rotor Internal rod Catalytic knob ADP + P i ATP MITOCHONDRIAL MATRIX Chemiosmosis couples the electron transport chain to ATP synthesis H+ H+ H+ H+ Protein complex of electron carriers Cyt c V Q ATP synthase FADH2 NADH 2 H+ + 1/2O2 H2O FAD NAD+ ADP + P i (carrying electrons from food) ATP H+ 1 Electron transport chain Oxidative phosphorylation 2 Chemiosmosis During cellular respiration, most energy flows in this sequence: glucose NADH electron transport chain proton-motive force ATP About 40% of the energy in a glucose molecule is transferred to ATP during cellular respiration, making about 38 ATP + 6 O2 6CO2 + 6H2O + 38 ATP ATP Yield per molecule of glucose at each stage of cellular respiration: Electron shuttles span membrane CYTOSOL 2 NADH Glycolysis Glucose 2 Pyruvate MITOCHONDRION 2 NADH or 2 FADH2 6 NADH 2 NADH 2 Acetyl CoA + 2 ATP Citric acid cycle + 2 ATP Maximum per glucose: About 36 or 38 ATP 2 FADH2 Oxidative phosphorylation: electron transport and chemiosmosis + about 32 or 34 ATP